Page 22 - Commercial Underwriting Mandates & Guidelines Binder Addendum
P. 22
Annexure A: Treaty Exclusions
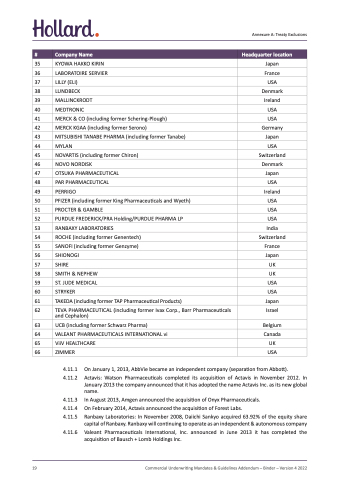
#
Company Name
Headquarter location
35 KYOWA HAKKO KIRIN
36 LABORATOIRE SERVIER
37 LILLY (ELI)
38 LUNDBECK Denmark
39 MALLINCKRODT Ireland
40 MEDTRONIC USA
Japan France USA
41 MERCK & CO (including former Schering-Plough)
42 MERCK KGAA (including former Serono)
43 MITSUBISHI TANABE PHARMA (including former Tanabe)
44 MYLAN USA
USA Germany Japan
45 NOVARTIS (including former Chiron)
46 NOVO NORDISK
47 OTSUKA PHARMACEUTICAL
48 PAR PHARMACEUTICAL
Switzerland Denmark Japan USA
49 PERRIGO Ireland
50 PFIZER (including former King Pharmaceuticals and Wyeth) USA
51 PROCTER & GAMBLE USA
52 PURDUE FREDERICK/PRA Holding/PURDUE PHARMA LP USA
53 RANBAXY LABORATORIES India
54 ROCHE (including former Genentech)
55 SANOFI (including former Genzyme)
56 SHIONOGI Japan
57 SHIRE UK
58 SMITH & NEPHEW UK
59 ST. JUDE MEDICAL USA
60 STRYKER USA
Switzerland France
61 TAKEDA (including former TAP Pharmaceutical Products)
62 TEVA PHARMACEUTICAL (including former Ivax Corp., Barr Pharmaceuticals and Cephalon)
63 UCB (including former Schwarz Pharma)
64 VALEANT PHARMACEUTICALS INTERNATIONAL vi
65 ViiV HEALTHCARE
66 ZIMMER USA
Japan Israel
Belgium Canada UK
4.11.1 4.11.2
4.11.3 4.11.4 4.11.5
4.11.6
On January 1, 2013, AbbVie became an independent company (separation from Abbott).
Actavis: Watson Pharmaceuticals completed its acquisition of Actavis in November 2012. In January 2013 the company announced that it has adopted the name Actavis Inc. as its new global name.
In August 2013, Amgen announced the acquisition of Onyx Pharmaceuticals.
On February 2014, Actavis announced the acquisition of Forest Labs.
Ranbaxy Laboratories: In November 2008, Daiichi Sankyo acquired 63.92% of the equity share capital of Ranbaxy. Ranbaxy will continuing to operate as an independent & autonomous company
Valeant Pharmaceuticals International, Inc. announced in June 2013 it has completed the acquisition of Bausch + Lomb Holdings Inc.
19
Commercial Underwriting Mandates & Guidelines Addendum – Binder – Version 4 2022