Page 22 - Gates-AnnualReport-2017
P. 22
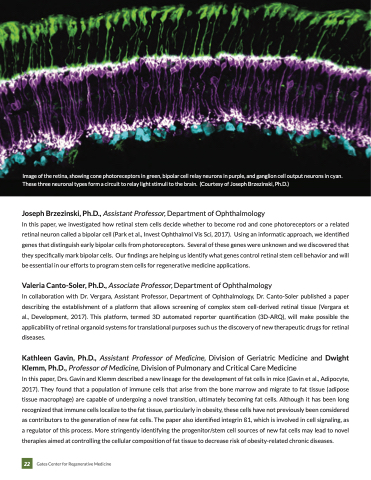
Image of the retina, showing cone photoreceptors in green, bipolar cell relay neurons in purple, and ganglion cell output neurons in cyan. These three neuronal types form a circuit to relay light stimuli to the brain. (Courtesy of Joseph Brzezinski, Ph.D.)
Joseph Brzezinski, Ph.D., Assistant Professor, Department of Ophthalmology
In this paper, we investigated how retinal stem cells decide whether to become rod and cone photoreceptors or a related retinal neuron called a bipolar cell (Park et al., Invest Ophthalmol Vis Sci, 2017). Using an informatic approach, we identified genes that distinguish early bipolar cells from photoreceptors. Several of these genes were unknown and we discovered that they specifically mark bipolar cells. Our findings are helping us identify what genes control retinal stem cell behavior and will be essential in our efforts to program stem cells for regenerative medicine applications.
Valeria Canto-Soler, Ph.D., Associate Professor, Department of Ophthalmology
In collaboration with Dr. Vergara, Assistant Professor, Department of Ophthalmology, Dr. Canto-Soler published a paper describing the establishment of a platform that allows screening of complex stem cell-derived retinal tissue (Vergara et al., Development, 2017). This platform, termed 3D automated reporter quantification (3D-ARQ), will make possible the applicability of retinal organoid systems for translational purposes such us the discovery of new therapeutic drugs for retinal diseases.
Kathleen Gavin, Ph.D., Assistant Professor of Medicine, Division of Geriatric Medicine and Dwight Klemm, Ph.D., Professor of Medicine, Division of Pulmonary and Critical Care Medicine
In this paper, Drs. Gavin and Klemm described a new lineage for the development of fat cells in mice (Gavin et al., Adipocyte, 2017). They found that a population of immune cells that arise from the bone marrow and migrate to fat tissue (adipose tissue macrophage) are capable of undergoing a novel transition, ultimately becoming fat cells. Although it has been long recognized that immune cells localize to the fat tissue, particularly in obesity, these cells have not previously been considered as contributors to the generation of new fat cells. The paper also identified integrin ß1, which is involved in cell signaling, as a regulator of this process. More stringently identifying the progenitor/stem cell sources of new fat cells may lead to novel therapies aimed at controlling the cellular composition of fat tissue to decrease risk of obesity-related chronic diseases.
22 Gates Center for Regenerative Medicine