Page 27 - eBook Version 8 Book 1 of 2 JUL 2022
P. 27
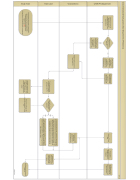
Study Team
Team Lead
Victoria Brown
UTSW PI’s Department
Cross Institutional Study process (Non-Department of Pediatrics)
UTSW department will determine who will complete regulatory start up procedures.
CMC support is needed for regulatory start up?
UTSW department will initiate regulatory start up
UTSW department will forward study regulatory documents to VB and request CTS.
Receive CTS and SOA
Forward fully executed SOA to VB and RA Finance at Children’s
As IRB approval is in process, study will begin transitioning to the study team.
Forward study information to appropriate department and Victoria Brown (VB): -PedSurg/Transplant Nadia Nassaj
-Ortho Surg (TBD)
-Urology Monica Villagomez (grants management)
Assigned coordinator will complete coordinator time sheet (CTS) within 5 business days of protocol receipt.
Assigned coordinator will discuss completed CTS with TL.
Study approval process will continue
YES
UTSW Department will forward request and all required documents to Victoria Brown (VB).
NO including completion and
submission of Lab Pre-Review form.
VB will email documents to Team Leads (TL).
Once approved VB will save and forward CTS to UTSW department.
TL will assign study to coordinator, and begin working on the CTS
Are Lab Pre-Review / Lab sheet forms complete?
NO
TL will review and forward completed CTS along with VB and schedule time to review as soon as possible but no later than 14 business days of protocol receipt. TL will work with assigned coordinator to complete Lab Pre-Review form/Lab sheet documents become available and schedule a time to review with VB.
Send Lab Pre- Review form with protocol and lab manual to lab for review.
YES
TL will review and forward completed CTS along with Lab Pre-Review/Lab sheets to VB and schedule time to review as soon as possible but no later than 14 business days of protocol receipt.
03/30/2020
Start Up