Page 30 - MRS Abstracts March 2023
P. 30
Results:
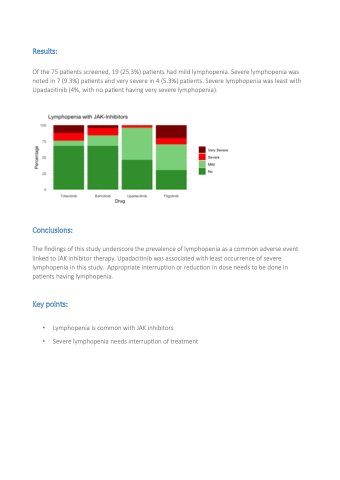
Of the 75 patients screened, 19 (25.3%) patients had mild lymphopenia. Severe lymphopenia was noted in 7 (9.3%) patients and very severe in 4 (5.3%) patients. Severe lymphopenia was least with Upadacitinib (4%, with no patient having very severe lymphopenia).
Conclusions:
The findings of this study underscore the prevalence of lymphopenia as a common adverse event linked to JAK inhibitor therapy. Upadacitinib was associated with least occurrence of severe lymphopenia in this study. Appropriate interruption or reduction in dose needs to be done in patients having lymphopenia.
Key points:
• Lymphopenia is common with JAK inhibitors
• Severe lymphopenia needs interruption of treatment