Page 1248 - Chemistry--atom first
P. 1248
1238
Appendix G
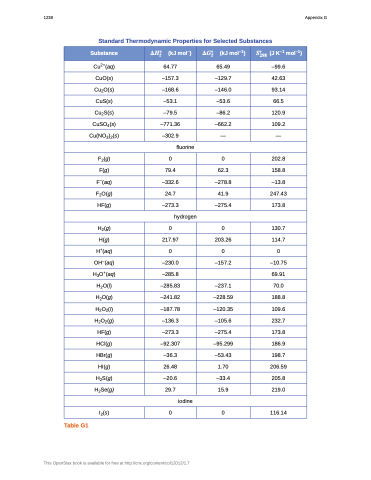
Standard Thermodynamic Properties for Selected Substances
Substance
���� (kJ mol–)
���� (kJ mol–1)
�� (J K–1 mol–1) ���
Cu2+(aq)
64.77
65.49
–99.6
CuO(s)
–157.3
–129.7
42.63
Cu2O(s)
–168.6
–146.0
93.14
CuS(s)
–53.1
–53.6
66.5
Cu2S(s)
–79.5
–86.2
120.9
CuSO4(s)
–771.36
–662.2
109.2
Cu(NO3)2(s)
–302.9
—
—
fluorine
F2(g)
0
0
202.8
F(g)
79.4
62.3
158.8
F–(aq)
–332.6
–278.8
–13.8
F2O(g)
24.7
41.9
247.43
HF(g)
–273.3
–275.4
173.8
hydrogen
H2(g)
0
0
130.7
H(g)
217.97
203.26
114.7
H+(aq)
0
0
0
OH–(aq)
–230.0
–157.2
–10.75
H3O+(aq)
–285.8
69.91
H2O(l)
–285.83
–237.1
70.0
H2O(g)
–241.82
–228.59
188.8
H2O2(l)
–187.78
–120.35
109.6
H2O2(g)
–136.3
–105.6
232.7
HF(g)
–273.3
–275.4
173.8
HCl(g)
–92.307
–95.299
186.9
HBr(g)
–36.3
–53.43
198.7
HI(g)
26.48
1.70
206.59
H2S(g)
–20.6
–33.4
205.8
H2Se(g)
29.7
15.9
219.0
iodine
I2(s) 0 0 116.14
Table G1
This OpenStax book is available for free at http://cnx.org/content/col12012/1.7