Page 110 - Geosystems An Introduction to Physical Geography 4th Canadian Edition
P. 110
74 part I The energy–atmosphere System
F cus Study 3.1 Pollution
Stratospheric Ozone Losses: A Continuing Health Hazard
More UVB radiation than ever before
is breaking through earth’s protective ozone layer, with detrimental effects
on human health, plants, and marine ecosystems. in humans, UVB causes skin cancer, cataracts (a clouding of the eye lens), and a weakening of the immune system. UVB alters plant physiology in complex ways that lead to decreased agricultural productivity. in marine eco- systems, scientists have documented 10% declines in phytoplankton produc- tivity in areas of ozone depletion around antarctica—these organisms are the primary producers that form the basis of the ocean’s food chain.
if all the ozone in our atmosphere were brought down to earth’s surface and compressed to surface pressure, the ozone layer would only be 3 mm thick. at an altitude of 29 km, where the ozone layer is densest, it contains only 1 part ozone per 4 million parts of air. yet this relatively thin layer was in steady-state equilibrium for several hundred million years, absorbing intense ultraviolet ra- diation and permitting life to proceed safely on earth.
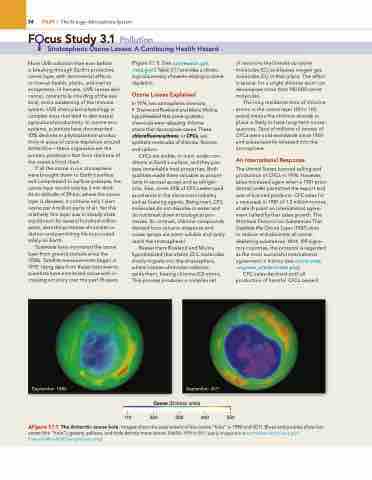
Scientists have monitored the ozone layer from ground stations since the 1920s. Satellite measurements began in 1978. Using data from these instruments, scientists have monitored ozone with in- creasing accuracy over the past 35 years
(Figure 3.1.1). (See ozonewatch.gsfc .nasa.gov/.) Table 3.1.1 provides a chrono- logical summary of events relating to ozone depletion.
Ozone Losses Explained
in 1974, two atmospheric chemists,
F. Sherwood rowland and Mario Molina, hypothesized that some synthetic chemicals were releasing chlorine atoms that decompose ozone. These chlorofluorocarbons, or CFCs, are synthetic molecules of chlorine, fluorine, and carbon.
CFCs are stable, or inert, under con- ditions at earth’s surface, and they pos- sess remarkable heat properties. Both qualities made them valuable as propel- lants in aerosol sprays and as refriger- ants. also, some 45% of CFCs were used as solvents in the electronics industry and as foaming agents. Being inert, CFC molecules do not dissolve in water and do not break down in biological pro- cesses. (in contrast, chlorine compounds derived from volcanic eruptions and ocean sprays are water soluble and rarely reach the stratosphere.)
researchers rowland and Molina hypothesized that stable CFC molecules slowly migrate into the stratosphere, where intense ultraviolet radiation
splits them, freeing chlorine (Cl) atoms. This process produces a complex set
of reactions that breaks up ozone molecules (O3) and leaves oxygen gas molecules (O2) in their place. The effect is severe, for a single chlorine atom can decompose more than 100 000 ozone molecules.
The long residence time of chlorine atoms in the ozone layer (40 to 100 years) means the chlorine already in place is likely to have long-term conse- quences. Tens of millions of tonnes of CFCs were sold worldwide since 1950 and subsequently released into the atmosphere.
An International Response
The United States banned selling and production of CFCs in 1978. However, sales increased again when a 1981 presi- dential order permitted the export and sale of banned products. CFC sales hit a new peak in 1987 of 1.2 million tonnes, at which point an international agree- ment halted further sales growth. The Montreal Protocol on Substances That Deplete the Ozone Layer (1987) aims
to reduce and eliminate all ozone- depleting substances. With 189 signa- tory countries, the protocol is regarded as the most successful international agreement in history (see ozone.unep .org/new_site/en/index.php).
CFC sales declined until all production of harmful CFCs ceased
September 1980
110
Ozone (Dobson units) 220 330 440
550
September 2011
▲Figure 3.1.1 The Antarctic ozone hole. images show the areal extent of the ozone “hole” in 1980 and 2011. Blues and purples show low ozone (the “hole”); greens, yellows, and reds denote more ozone. [naSa; 1979 to 2011 yearly images are at earthobservatory.nasa.gov/ Features/WorldOfChange/ozone.php.]