Page 113 - Geosystems An Introduction to Physical Geography 4th Canadian Edition
P. 113
Chapter 3 earth’s Modern atmosphere 77
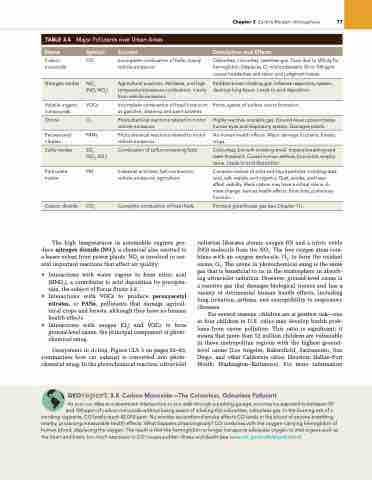
TABLE 3.4 Major Pollutants over Urban Areas
Name
Symbol
Sources
Description and Effects
Carbon CO monoxide
Volatile organic VOCs compounds
Peroxyacetyl Pans nitrates
Particulate PM matter
incomplete combustion of fuels, mainly vehicle emissions
incomplete combustion of fossil fuels such as gasoline; cleaning and paint solvents
Photochemical reactions related to motor vehicle emissions
industrial activities, fuel combustion, vehicle emissions, agriculture
nitrogen oxides
nOx
(nO, nO2)
agricultural practices, fertilizers, and high temperature/pressure combustion, mainly from vehicle emissions
reddish-brown choking gas. inflames respiratory system, destroys lung tissue. leads to acid deposition.
Ozone
O3
Photochemical reactions related to motor vehicle emissions
Highly reactive, unstable gas. ground-level ozone irritates human eyes and respiratory system. Damages plants.
Sulfur oxides
SOx
(SO2, SO3)
Combustion of sulfur-containing fuels
Colourless, but with irritating smell. impairs breathing and taste threshold. Causes human asthma, bronchitis, emphy- sema. leads to acid deposition.
Carbon dioxide
CO2
Complete combustion of fossil fuels
Principal greenhouse gas (see Chapter 11).
The high temperatures in automobile engines pro- duce nitrogen dioxide (NO2), a chemical also emitted to a lesser extent from power plants. NO2 is involved in sev- eral important reactions that affect air quality:
• Interactions with water vapour to form nitric acid (HNO3), a contributor to acid deposition by precipita- tion, the subject of Focus Study 3.2.
• Interactions with VOCs to produce peroxyacetyl nitrates, or PANs, pollutants that damage agricul- tural crops and forests, although they have no human health effects.
• Interactions with oxygen (O2) and VOCs to form ground-level ozone, the principal component of photo- chemical smog.
Geosystems in Action, Figure GIA 3 on pages 82–83, summarizes how car exhaust is converted into photo- chemical smog. In the photochemical reaction, ultraviolet
Odourless, colourless, tasteless gas. Toxic due to affinity for hemoglobin. Displaces O2 in bloodstream; 50 to 100 ppm causes headaches and vision and judgment losses.
Prime agents of surface ozone formation.
no human health effects. Major damage to plants, forests, crops.
Complex mixture of solid and liquid particles including dust, soot, salt, metals, and organics. Dust, smoke, and haze affect visibility. Black carbon may have a critical role in cli- mate change. Various health effects: bronchitis, pulmonary function.
radiation liberates atomic oxygen (O) and a nitric oxide (NO) molecule from the NO2. The free oxygen atom com- bines with an oxygen molecule, O2, to form the oxidant ozone, O3. The ozone in photochemical smog is the same gas that is beneficial to us in the stratosphere in absorb- ing ultraviolet radiation. However, ground-level ozone is a reactive gas that damages biological tissues and has a variety of detrimental human health effects, including lung irritation, asthma, and susceptibility to respiratory illnesses.
For several reasons, children are at greatest risk—one in four children in U.S. cities may develop health prob- lems from ozone pollution. This ratio is significant; it means that more than 12 million children are vulnerable in those metropolitan regions with the highest ground- level ozone (Los Angeles, Bakersfield, Sacramento, San Diego, and other California cities; Houston; Dallas–Fort Worth; Washington–Baltimore). For more information
Georeport 3.5 Carbon Monoxide—The Colourless, Odourless Pollutant
as your car idles at a downtown intersection or you walk through a parking garage, you may be exposed to between 50 and 100 ppm of carbon monoxide without being aware of inhaling this colourless, odourless gas. in the burning ash of a smoking cigarette, CO levels reach 42 000 ppm. no wonder secondhand smoke affects CO levels in the blood of anyone breathing
nearby, producing measurable health effects. What happens physiologically? CO combines with the oxygen-carrying hemoglobin of human blood, displacing the oxygen. The result is that the hemoglobin no longer transports adequate oxygen to vital organs such as the heart and brain; too much exposure to CO causes sudden illness and death (see www.cdc.gov/nceh/airpollution/.)