Page 219 - Geosystems An Introduction to Physical Geography 4th Canadian Edition
P. 219
Chapter 7 Water and Atmospheric Moisture 183
Phase Changes and Heat Exchange
For water to change from one state to another, heat energy must be added to it or released from it. The amount of heat energy absorbed or released must be sufficient to affect the hydrogen bonds between molecules. This relation be tween water and heat energy is important to atmospheric processes. In fact, the heat exchanged between physical states of water provides more than 30% of the energy that powers the general circulation of the atmosphere.
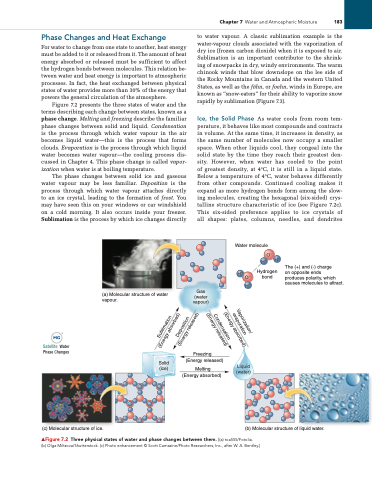
Figure 7.2 presents the three states of water and the terms describing each change between states, known as a phase change. Melting and freezing describe the familiar phase changes between solid and liquid. Condensation is the process through which water vapour in the air becomes liquid water—this is the process that forms clouds. Evaporation is the process through which liquid water becomes water vapour—the cooling process dis cussed in Chapter 4. This phase change is called vapor- ization when water is at boiling temperature.
The phase changes between solid ice and gaseous water vapour may be less familiar. Deposition is the process through which water vapour attaches directly to an ice crystal, leading to the formation of frost. You may have seen this on your windows or car windshield on a cold morning. It also occurs inside your freezer. Sublimation is the process by which ice changes directly
to water vapour. A classic sublimation example is the watervapour clouds associated with the vaporization of dry ice (frozen carbon dioxide) when it is exposed to air. Sublimation is an important contributor to the shrink ing of snowpacks in dry, windy environments. The warm chinook winds that blow downslope on the lee side of the Rocky Mountains in Canada and the western United States, as well as the föhn, or foehn, winds in Europe, are known as “snoweaters” for their ability to vaporize snow rapidly by sublimation (Figure 7.3).
Ice, the Solid Phase As water cools from room tem perature, it behaves like most compounds and contracts in volume. At the same time, it increases in density, as the same number of molecules now occupy a smaller space. When other liquids cool, they congeal into the solid state by the time they reach their greatest den sity. However, when water has cooled to the point of greatest density, at 4°C, it is still in a liquid state. Below a temperature of 4°C, water behaves differently from other compounds. Continued cooling makes it expand as more hydrogen bonds form among the slow ing molecules, creating the hexagonal (sixsided) crys talline structure characteristic of ice (see Figure 7.2c). This sixsided preference applies to ice crystals of all shapes: plates, columns, needles, and dendrites
(a) Molecular structure of water vapour.
Gas (water vapour)
Freezing (Energy released)
Melting (Energy absorbed)
Water molecule
H+
O–
H+ H+
– Hydrogen O bond
H+
The (+) and (-) charge
on opposite ends
produces polarity, which causes molecules to attract.
Satellite Water Phase Changes
Solid (ice)
Liquid (water)
(c) Molecular structure of ice.
(b) Molecular structure of liquid water.
▲Figure 7.2 Three physical states of water and phase changes between them. [(a) toa555/Fotolia.
(b) Olga Miltsova/Shutterstock. (c) Photo enhancement © Scott Camazine/Photo Researchers, inc., after W. A. Bentley.]
Vaporization/ evaporation
(Energy absorbed)
Condensation (Energy released)
Sublimation Deposition
(Energy absorbed) (Energy released)