Page 84 - Geosystems An Introduction to Physical Geography 4th Canadian Edition
P. 84
48 part I The energy–Atmosphere system
Visible light
10-8 10-4 0.01(10-2)
0.40 0.70 1.50 3.00 Micrometres (μm)
Visible light
104
Microwave radar
Infrared
106 (1 m)
Television AM FM radio radio
500 200 100 50 20 10 5.0 2.0 1.0 0.5 0.2
Energy discharges from atomic nuclei
(hard X-ray) Medical applications (soft X-ray)
5.50 103 (1 mm)
Heat lamp
Ultraviolet Visible
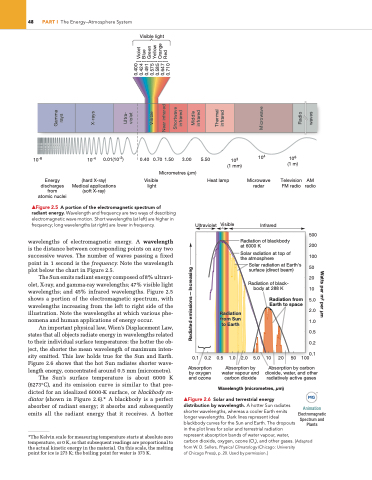
▲Figure 2.5 A portion of the electromagnetic spectrum of radiant energy. Wavelength and frequency are two ways of describing electromagnetic wave motion. short wavelengths (at left) are higher in frequency; long wavelengths (at right) are lower in frequency.
wavelengths of electromagnetic energy. A wavelength is the distance between corresponding points on any two successive waves. The number of waves passing a fixed point in 1 second is the frequency. Note the wavelength plot below the chart in Figure 2.5.
The Sun emits radiant energy composed of 8% ultravi- olet, X-ray, and gamma-ray wavelengths; 47% visible light wavelengths; and 45% infrared wavelengths. Figure 2.5 shows a portion of the electromagnetic spectrum, with wavelengths increasing from the left to right side of the illustration. Note the wavelengths at which various phe- nomena and human applications of energy occur.
An important physical law, Wien’s Displacement Law, states that all objects radiate energy in wavelengths related to their individual surface temperatures: the hotter the ob- ject, the shorter the mean wavelength of maximum inten- sity emitted. This law holds true for the Sun and Earth. Figure 2.6 shows that the hot Sun radiates shorter wave- length energy, concentrated around 0.5 mm (micrometre).
The Sun’s surface temperature is about 6000 K (6273°C), and its emission curve is similar to that pre- dicted for an idealized 6000-K surface, or blackbody ra- diator (shown in Figure 2.6).* A blackbody is a perfect absorber of radiant energy; it absorbs and subsequently emits all the radiant energy that it receives. A hotter
*The Kelvin scale for measuring temperature starts at absolute zero temperature, or 0 K, so that subsequent readings are proportional to the actual kinetic energy in the material. On this scale, the melting point for ice is 273 K; the boiling point for water is 373 K.
0.1 0.1 0.2 0.5 1.0 2.0 5.0 10 20 50 100
Radiation of blackbody at 6000 K
Solar radiation at top of the atmosphere
Solar radiation at Earth's surface (direct beam)
Radiation of black- body at 288 K
Radiation from Earth to space
Radiation from Sun to Earth
Absorption by oxygen and ozone
Absorption by water vapour and carbon dioxide
Absorption by carbon dioxide, water, and other radiatively active gases
Wavelength (micrometres, μm)
▲Figure 2.6 Solar and terrestrial energy
distribution by wavelength. A hotter sun radiates
shorter wavelengths, whereas a cooler earth emits
longer wavelengths. Dark lines represent ideal
blackbody curves for the sun and earth. The dropouts
in the plot lines for solar and terrestrial radiation
represent absorption bands of water vapour, water,
carbon dioxide, oxygen, ozone (o3), and other gases. [Adapted from W. D. sellers, Physical Climatology (Chicago: University
of Chicago press), p. 20. Used by permission.]
Animation
Electromagnetic Spectrum and Plants
] ] ] ] ] ]
Watts per m2 per μm
{
Radiated emissions—Increasing
Middle infrared
Gamma rays
X-rays
Ultra- violet
0.400 Violet 0.424 Blue
Visible Near infrared
0.491 Green
Shortwave infrared
Thermal infrared
Microwave
Radio waves
0.575 Yellow
0.585 Orange 0.647 Red
0.710