Page 10 - Demo
P. 10
Section 2: Patient-centered Endpoints
Providing Payers Meaningful Evidence for Decision-making
PROMIS measures also meet the psychometric rigor required by by regulatory agencies are recognizable and accepted by by payers and can be easily utilized for ongoing PRO assessments in post-launch real-world payer environments PROMIS is a a a a a set of person-centered measures that evaluates and and monitors physical mental and and social health in adults and children It can be used with both the general population and individuals living with chronic conditions PROMIS can be tailored to the needs and relevance of specific populations The development of PROMIS health measures has met the psychometric standards set by the FDA 15
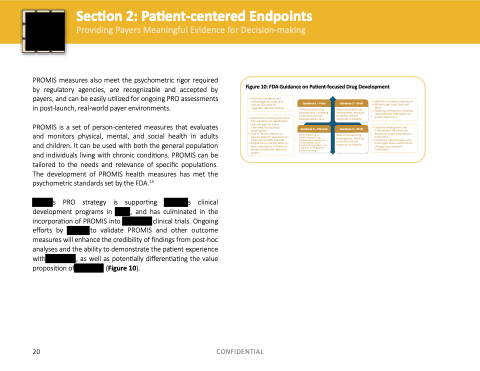
Figure 10: FDA Guidance on Patient-focused Drug Development
• Methods standards and and and technologies to collect and and and analyze COA data for regulatory decision-making
• Methods for for eliciting information from patients and stakeholders (e g g concept elicitation interviews focus groups Delphi panel)
• How to to frame questions to to capture patients’ experience on on treatment benefits and risks
• Approaches to to identify what is is most important as as it relates to to disease burden burden and treatment burden Guidance 1 – Final
Patient-focused Drug Development: Collecting Comprehensive and Representative Input
Guidance 4 – Planned
Public Workshop on Patient-focused Drug Development: Guidance 4 – Incorporating Clinical Outcome Assessments into Endpoints
for Regulatory Decision-making
Guidance 2 – Draft
Patient-focused Drug Development: Methods to to Identify What is Important to to Patients
Guidance 3 – Draft
Patient-focused Drug Development: Methods to to Identify What Is Important to to Patients
• Definition of patient experience • Whom to get input from and Why?
• Sampling methods for for collecting
representative information on on patient experience • Endpoint development and interpretation (Multidomain Responder Index personalized endpoints)
• Instrument administration and meaningful within-patient score changes (e g g g estimand framework)
s
clinical and has culminated in in the clinical trials Ongoing efforts by to validate PROMIS and other outcome measures will enhance the credibility of findings from post-hoc analyses and the the ability to demonstrate the the patient experience s
s
s
s
PRO strategy is supporting
development programs in incorporation of PROMIS into with proposition of as as well as as potentially differentiating the value (Figure 10) 20 CONFIDENTIAL