Page 8 - Research Compliance Welcome Package (1.4.19)
P. 8
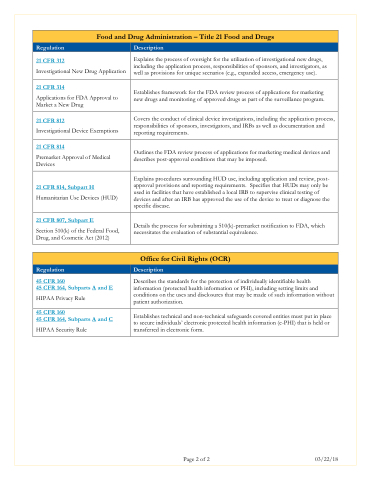
Food and Drug Administration – Title 21 Food and Drugs
Regulation
Description
21 CFR 312
Investigational New Drug Application
Explains the process of oversight for the utilization of investigational new drugs, including the application process, responsibilities of sponsors, and investigators, as well as provisions for unique scenarios (e.g., expanded access, emergency use).
21 CFR 314
Applications for FDA Approval to Market a New Drug
Establishes framework for the FDA review process of applications for marketing new drugs and monitoring of approved drugs as part of the surveillance program.
21 CFR 812
Investigational Device Exemptions
Covers the conduct of clinical device investigations, including the application process, responsibilities of sponsors, investigators, and IRBs as well as documentation and reporting requirements.
21 CFR 814
Premarket Approval of Medical Devices
Outlines the FDA review process of applications for marketing medical devices and describes post-approval conditions that may be imposed.
21 CFR 814, Subpart H
Humanitarian Use Devices (HUD)
Explains procedures surrounding HUD use, including application and review, post- approval provisions and reporting requirements. Specifies that HUDs may only be used in facilities that have established a local IRB to supervise clinical testing of devices and after an IRB has approved the use of the device to treat or diagnose the specific disease.
21 CFR 807, Subpart E
Section 510(k) of the Federal Food, Drug, and Cosmetic Act (2012)
Details the process for submitting a 510(k)-premarket notification to FDA, which necessitates the evaluation of substantial equivalence.
Office for Civil Rights (OCR)
Regulation
Description
45 CFR 160
45 CFR 164, Subparts A and E
HIPAA Privacy Rule
Describes the standards for the protection of individually identifiable health information (protected health information or PHI), including setting limits and conditions on the uses and disclosures that may be made of such information without patient authorization.
45 CFR 160
45 CFR 164, Subparts A and C
HIPAA Security Rule
Establishes technical and non-technical safeguards covered entities must put in place to secure individuals’ electronic protected health information (e-PHI) that is held or transferred in electronic form.
Page 2 of 2 03/22/18