Page 120 - Instrumen Soal HOTS
P. 120
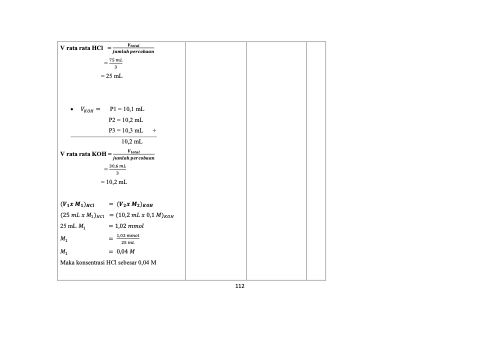
V rata rata HCl =
= 75 𝑚𝐿 3
•
= 25 mL
P2 = 10,2 mL
P3 = 10,3 mL +
10,2 mL
= 10,2 mL
= (𝑽𝟐𝒙 𝑴𝟐)𝑲𝑶𝑯
𝑉 = P1 = 10,1 mL 𝐾𝑂𝐻
V rata rata KOH =
𝑽𝒕𝒐𝒕𝒂𝒍
𝒋𝒖𝒎𝒍𝒂𝒉 𝒑𝒆𝒓𝒄𝒐𝒃𝒂𝒂𝒏
= 30,6 𝑚𝐿 3
(𝑽𝟏𝒙 𝑴𝟏)𝑯𝑪𝒍
(25𝑚𝐿𝑥𝑀 ) =(10,2𝑚𝐿𝑥0,1𝑀)
1 𝐻𝐶𝑙 𝐾𝑂𝐻
25 mL 𝑀 = 1,02 𝑚𝑚𝑜𝑙 1
𝑀 1
= 1,02𝑚𝑚𝑜𝑙 25 𝑚𝐿
𝑀 = 0,04𝑀 1
𝑽𝒕𝒐𝒕𝒂𝒍
𝒋𝒖𝒎𝒍𝒂𝒉 𝒑𝒆𝒓𝒄𝒐𝒃𝒂𝒂𝒏
Maka konsentrasi HCl sebesar 0,04 M
112