Page 3 - Roche RIM presentation
P. 3
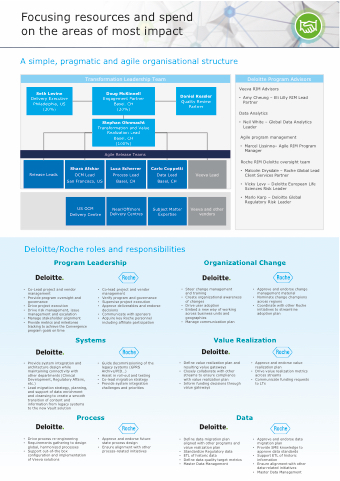
Focusing resources and spend on the areas of most impact
A simple, pragmatic and agile organisational structure
Deloitte Program Advisors
Veeva RIM Advisors
• Amy Cheung – Eli Lilly RIM Lead Partner
Data Analytics
• Neil White – Global Data Analytics Leader
Agile program management
• Marcel Lissinna– Agile RIM Program Manager
Roche RIM Deloitte oversight team
• MalcolmDrysdale–RocheGlobalLead Client Services Partner
• VickyLevy–DeloitteEuropeanLife Sciences Risk Leader
• Marlo Karp – Deloitte Global Regulatory Risk Leader
Seth Levine
Delivery Executive Philadelphia, US (20%)
Transformation Leadership Team
Doug McKinnell
Engagement Partner Basel, CH (20%)
Daniel Ressler
Quality Review Partner
Stephan Ohnmacht
Transformation and Value Realization Lead Basel, CH (100%)
Agile Release Teams
Release Leads
Shara Afshar
OCM Lead San Francisco, US
Deloitte/Roche roles and responsibilities
Program Leadership
Organizational Change
Luca Scherrer
Process Lead Basel, CH
Carlo Coppetti
Data Lead Basel, CH
Veeva Lead
US OCM Delivery Centre
Near/Offshore Delivery Centres
Subject Matter Expertise
Veeva and other vendors
• Co-Lead project and vendor management
• Provide program oversight and governance
• Drive project execution
• Drive risk management, issue
management and escalation
• Manage stakeholder alignment
• Provide metrics and milestones
tracking to achieve the Convergence program goals on time
• Co-lead project and vendor management
• Verify program and governance
• Supervise project execution
• Approve deliverables and endorse
decisions
• Communicate with sponsors • Acquire key Roche personnel
including affiliate participation
• •
• •
•
Steer change management
and training
Create organizational awareness of changes
Drive user adoption
Embed a new way of working across business units and geographies
Manage communication plan
• Approve and endorse change management material
• Nominate change champions across regions
• Coordinate with other Roche initiatives to streamline adoption plan
Systems
Value Realization
• Provide system integration and architecture design while
maintaining connectivity with
other departments (Clinical Development, Regulatory Affairs,
etc.) •
• Lead migration strategy, planning, and support of data enrichment and cleansing to create a smooth transition of content and information from legacy systems to the new Vault solution
Guide decommissioning of the legacy systems (GPRS Archive/PID...)
Assist in roll-out and testing Co-lead migration strategy Provide system integration challenges and priorities
• •
•
•
• • • •
Define value realization plan and • resulting value gateways
Closely collaborate with other • streams to ensure compliance
with value realization plan • Inform funding decisions through
value gateways
Data
Define data migration plan • aligned with other programs and
value realization plan • Standardize Regulatory data
ETL of historic data • Define data quality target metrics Master Data Management •
Approve and endorse value realization plan
Drive value realization metrics across streams
Communicate funding requests to LTs
Approve and endorse data migration plan
Provide SME knowledge to approve data standards Support ETL of historic information
Ensure alignment with other
Process
•
• •
• Drive process re-engineering
• Requirements gathering to design
global, harmonized processes
• Support out-of-the box
configuration and implementation of Veeva solutions
• Approve and endorse future state process design
• Ensure alignment with other process-related initiatives
data-related initiatives
• Master Data Management