Page 184 - 2019秋季手冊內頁-ebook測試
P. 184
衛生福利部雙和醫院(委託臺北醫學大學興建經營)
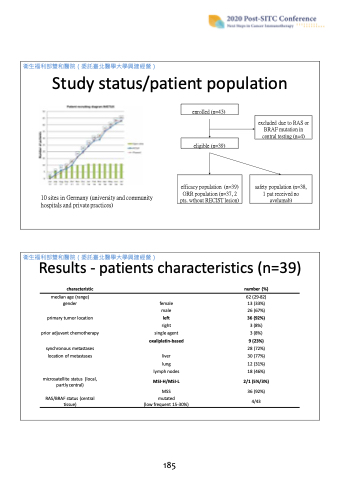
Study status/patient population
10 sites in Germany (university and community hospitals and private practices)
enrolled (n=43)
excluded due to RAS or BRAF mutation in central testing (n=4)
eligible (n=39)
efficacy population (n=39) ORR population (n=37, 2 pts. wthout RECIST lesion)
safety population (n=38, 1 pat received no avelumab)
衛生福利部雙和醫院(委託臺北醫學大學興建經營)
Results - patients characteristics (n=39)
characteristic
median age (range) gender
primary tumor location prior adjuvant chemotherapy
synchronous metastases location of metastases
microsatellite status (local, partly central)
RAS/BRAF status (central tissue)
female male
left
right single agent oxaliplatin-based
liver
lung lymph nodes
MSI-H/MSI-L
MSS
mutated
(low frequent 15-30%)
number (%)
62 (29-82) 13 (33%) 26 (67%) 36 (92%) 3 (8%)
3 (8%)
9 (23%)
28 (72%) 30 (77%) 12 (31%) 18 (46%)
2/1 (5%/3%)
36 (92%) 4/43
185