Page 197 - 2019秋季手冊內頁-ebook測試
P. 197
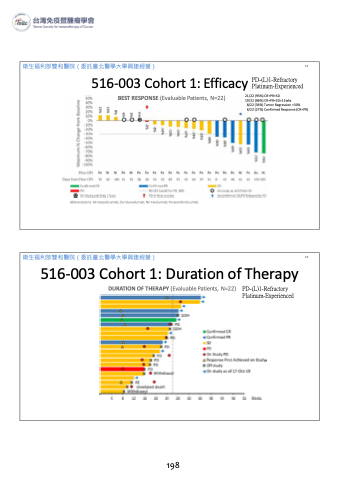
衛生福利部雙和醫院(委託臺北醫學大學興建經營)
15
8/22 (36%) Tumor Regression >30% 6/22 (27%) Confirmed Response (CR+PR)
516-003 Cohort 1: Efficacy PD-(L)1-Refractory Platinum-Experienced
BEST RESPONSE (Evaluable Patients, N=22)
21/22 (95%) CR+PR+SD
19/22 (86%) CR+PR+SD>12wks
Abbreviations: At=atezolizumab; Du=durvalumab; Ni=nivolumab; Pe=pembrolizumab
衛生福利部雙和醫院(委託臺北醫學大學興建經營)
16
516-003 Cohort 1: Duration of Therapy
DURATION OF THERAPY (Evaluable Patients, N=22) PD-(L)1-Refractory Platinum-Experienced
198