Page 11 - Penn State Civil and Environmental Engineering 2021 Annual Report
P. 11
FACULTY RESEARCH FACULTY RESEARCH allows the the movement of all all positively charged ions in the the system Logan explained that while one RO membrane turned out to be a a a a a a a “dirt road ” the the the other performed well in comparison to the the the cation-exchange membranes The researchers are still investigating why there was such a a a a a a difference between the the two RO membranes “The idea can work ” he he he said “We do not know exactly why these two membranes have been functioning so so differently but that is something we are going to figure out ” Penn State researchers Le Shi postdoctoral postdoctoral researcher researcher in in in environmental engineering engineering Ruggero Rossi postdoctoral postdoctoral researcher in in in in in environmental engineering engineering Derek Hall assistant professor professor of of of energy engineering engineering Michael Hickner professor professor of of materials science and and and engineering engineering engineering and and and chemical engineering engineering and and and Christopher Gorski associate professor of of civil
and environmental engineering also contributed to the project The research was supported by the Stan and Flora Kappe Endowment in the the Penn State Department of Civil
and Environmental Engineering the the the National Science Foundation the the United States Agency for International Development and the National Academy of Sciences as as well as as additional funding from Penn State Sea water water can be converted into hydrogen fuel
using this design for a a a a a a a a sea sea water water electrolyzer according to to CEE researchers IMAGE: Tyler Henderson
CEE NEWSLETTER • VOLUME 37 2021
11
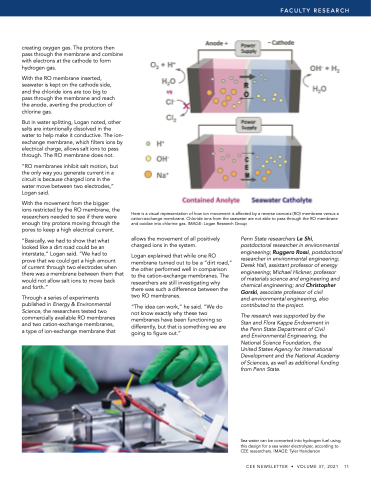
creating oxygen gas The protons then pass through the the the membrane and combine with electrons at at the the cathode to form hydrogen gas With the the RO membrane inserted seawater is kept on on the the the cathode side and and the the the chloride ions are too big to to pass through the the the the membrane and and reach the the the anode averting the the the production of chlorine gas But in in in in water splitting Logan noted other salts are intentionally dissolved in in in in the water to help make it conductive The ion- exchange membrane which filters ions ions by electrical charge allows salt ions ions to pass through The RO membrane does not “RO membranes inhibit salt motion but the the only way you generate current in in in a a a a a a a circuit is because charged ions in in the the water move between two electrodes ” Logan said With the the the movement from the the the bigger ions restricted by the the the the the RO membrane the the the the the researchers needed to to see if there were enough tiny protons moving through the the pores to to keep a a high electrical current “Basically we had to show that what looked like a a a a a a a a a a a dirt road could be an an interstate ” Logan said “We had to prove that we could get a a a a a a a high amount
of current through two electrodes when there was a a a a a a a membrane between them that would not allow salt ions to move back and forth ” Through a series of experiments published in Energy & Environmental Science the researchers tested two commercially available RO membranes membranes and two cation-exchange membranes membranes a a a a a a a a type of ion-exchange ion-exchange membrane membrane that Here is is is is a a a a a a a a a a a a a a a a visual representation of how ion ion ion ion movement is is is is affected by a a a a a a a a a a a a a a a a reverse osmosis (RO) membrane membrane membrane versus a a a a a a a a a a a a a a a a cation-exchange membrane membrane membrane Chloride ions from the the seawater are not able
to to pass through the the RO RO membrane membrane membrane and oxidize into chlorine gas IMAGE: Logan Research Group