Page 5 - E-Science Prep.2 2024-2025 t1 Unit1_Neat
P. 5
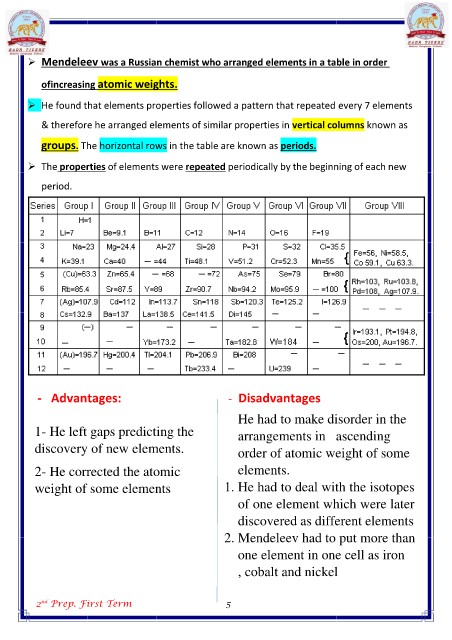
? Mendeleev was a Russian chemist who arranged elements in a table in order
ofincreasing atomic weights.
? He found that elements properties followed a pattern that repeated every 7 elements
& therefore he arranged elements of similar properties in vertical columns known as
groups. The horizontal rows in the table are known as periods.
? The properties of elements were repeated periodically by the beginning of each new
period.
- Advantages: - Disadvantages
1- He left gaps predicting the
discovery of new elements. He had to make disorder in the
2- He corrected the atomic arrangements in ascending
weight of some elements order of atomic weight of some
elements.
2nd Prep. First Term 1. He had to deal with the isotopes
of one element which were later
discovered as different elements
2. Mendeleev had to put more than
one element in one cell as iron
, cobalt and nickel
5