Page 108 - Org 3 theoritical book 2024-25
P. 108
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
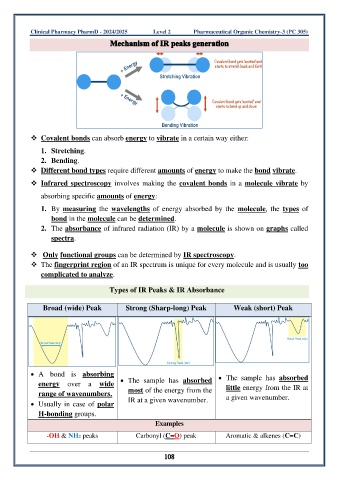
❖ Covalent bonds can absorb energy to vibrate in a certain way either:
1. Stretching.
2. Bending.
❖ Different bond types require different amounts of energy to make the bond vibrate.
❖ Infrared spectroscopy involves making the covalent bonds in a molecule vibrate by
absorbing specific amounts of energy:
1. By measuring the wavelengths of energy absorbed by the molecule, the types of
bond in the molecule can be determined.
2. The absorbance of infrared radiation (IR) by a molecule is shown on graphs called
spectra.
❖ Only functional groups can be determined by IR spectroscopy.
❖ The fingerprint region of an IR spectrum is unique for every molecule and is usually too
complicated to analyze.
Types of IR Peaks & IR Absorbance
Broad (wide) Peak Strong (Sharp-long) Peak Weak (short) Peak
• A bond is absorbing • The sample has absorbed
energy over a wide • The sample has absorbed little energy from the IR at
range of wavenumbers. most of the energy from the a given wavenumber.
• Usually in case of polar IR at a given wavenumber.
H-bonding groups.
Examples
-OH & NH2 peaks Carbonyl (C=O) peak Aromatic & alkenes (C=C)