Page 132 - Genius Kidz Prudence Learning Sc-8
P. 132
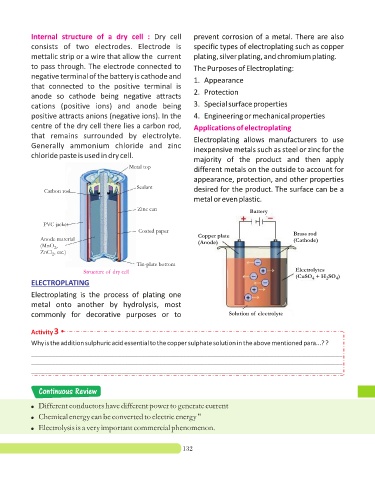
Internal structure of a dry cell : Dry cell prevent corrosion of a metal. There are also
consists of two electrodes. Electrode is specific types of electroplating such as copper
mettalic strip or a wire that allow the current plating, silver plating, and chromium plating.
to pass through. The electrode connected to The Purposes of Electroplating:
negative terminal of the battery is cathode and
1. Appearance
that connected to the positive terminal is
2. Protection
anode so cathode being negative attracts
cations (positive ions) and anode being 3. Special surface properties
positive attracts anions (negative ions). In the 4. Engineering or mechanical properties
centre of the dry cell there lies a carbon rod, Applications of electroplating
that remains surrounded by electrolyte.
Electroplating allows manufacturers to use
Generally ammonium chloride and zinc inexpensive metals such as steel or zinc for the
chloride paste is used in dry cell.
majority of the product and then apply
Metal top
different metals on the outside to account for
appearance, protection, and other properties
Sealant
Carbon rod desired for the product. The surface can be a
metal or even plastic.
Zinc can
Battery
PVC jacket
Coated paper
Copper plate Brass rod
Anode material (Cathode)
(Anode)
(MnO ,
2
ZnCl , etc.)
2
Tin-plate bottom
Structure of dry cell Electrolytes
(CuSO + H SO )
4
4
2
ELECTROPLATING
Electroplating is the process of plating one
metal onto another by hydrolysis, most
commonly for decorative purposes or to Solution of electrolyte
Activity 3
Why is the addition sulphuric acid essential to the copper sulphate solution in the above mentioned para...? ?
__________________________________________________________________________________________
__________________________________________________________________________________________
__________________________________________________________________________________________
! Different conductors have different power to generate current
! Chemical energy can be converted to electric energy "
! Electrolysis is a very important commercial phenomenon.
132