Page 55 - Ripples SCIENCE 7 - TEJPUR Edition 2024 Answer Key
P. 55
6. What happens when:
(a) an acid reacts with a base
Ans. (a) When an acid reacts with a base a neutralization reaction occurs, producing a
salt and water.
Acid + Base Salt + water
For example, when hydrochloric acid (HCl) reacts with sodium hydroxide
(NaOH), sodium chloride (NaCl) and water (H O) are formed.
2
HCl + NaOH NaCl + H O.
2
(b) blue litmus is added to vinegar
Ans. When blue litmus paper is added to vinegar, it turns red. This happens because
vinegar is an acidic substance, primarily composed of acetic acid, which has a pH less
than 7.
(c) lemon juice is added to baking soda
Ans. When lemon juice is added to baking soda, a chemical reaction occurs in which
carbon dioxide, water and a salt is produced.
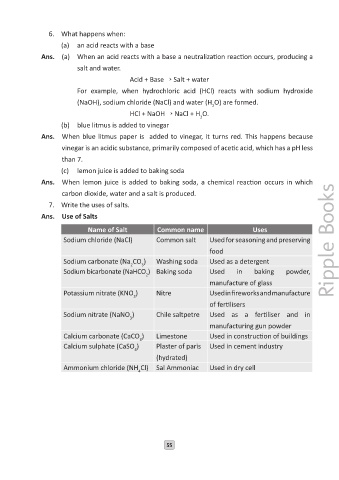
7. Write the uses of salts.
Ans. Use of Salts
Name of Salt Common name Uses
Sodium chloride (NaCl) Common salt Used for seasoning and preserving
food
Sodium carbonate (Na CO ) Washing soda Used as a detergent
2
3
Sodium bicarbonate (NaHCO ) Baking soda Used in baking powder,
2
manufacture of glass
Potassium nitrate (KNO ) Nitre Used in fireworks and manufacture
3
of fertilisers
Sodium nitrate (NaNO ) Chile saltpetre Used as a fertiliser and in
3
manufacturing gun powder
Calcium carbonate (CaCO ) Limestone Used in construction of buildings
3
Calcium sulphate (CaSO ) Plaster of paris Used in cement industry
4
(hydrated)
Ammonium chloride (NH Cl) Sal Ammoniac Used in dry cell
4
55