Page 60 - Mass General Brigham - Innovators Guide 1.2
P. 60
60 INNO V AT O RS GUIDE | M ASS GENERAL BRIGH AM
Resources | funding
11
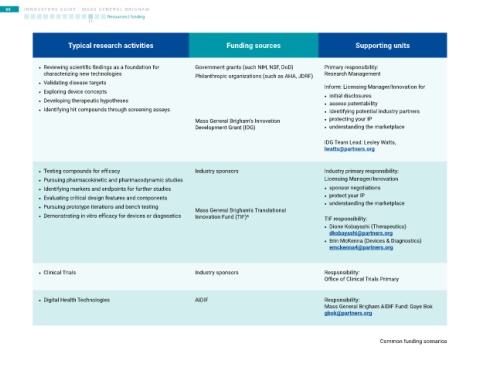
Typical research activities Funding sources Supporting units
• Reviewing scientific findings as a foundation for Government grants (such NIH, NSF, DoD) Primary responsibility:
characterizing new technologies Philanthropic organizations (such as AHA, JDRF) Research Management
• Validating disease targets
Inform: Licensing Manager/Innovation for
• Exploring device concepts • initial disclosures
• Developing therapeutic hypotheses • assess patentability
• Identifying hit compounds through screening assays • identifying potential industry partners
Mass General Brigham’s Innovation • protecting your IP
Development Grant (IDG) • understanding the marketplace
IDG Team Lead: Lesley Watts,
lwatts@partners.org
• Testing compounds for efficacy Industry sponsors Industry primary responsibility:
• Pursuing pharmacokinetic and pharmacodynamic studies Licensing Manager/Innovation
• Identifying markers and endpoints for further studies • sponsor negotiations
• Evaluating critical design features and components • protect your IP
• Pursuing prototype iterations and bench testing Mass General Brigham’s Translational • understanding the marketplace
• Demonstrating in vitro efficacy for devices or diagnostics Innovation Fund (TIF)* TIF responsibility:
• Dione Kobayashi (Therapeutics)
dkobayashi@partners.org
• Erin McKenna (Devices & Diagnostics)
emckenna4@partners.org
• Clinical Trials Industry sponsors Responsibility:
Office of Clinical Trials Primary
• Digital Health Technologies AIDIF Responsibility:
Mass General Brigham AIDIF Fund: Gaye Bok
gbok@partners.org
Common funding scenarios