Page 11 - Introduction QC
P. 11
GMP Training – Introduction to Quality Control (QC) by www.gmpsop.com
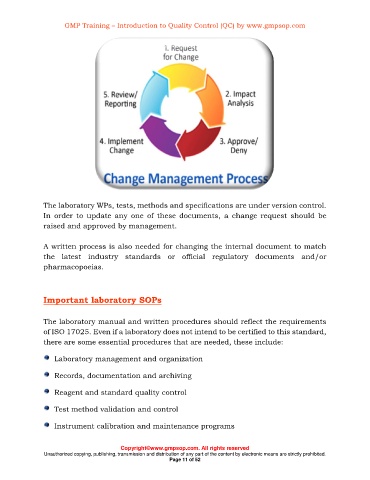
The laboratory WPs, tests, methods and specifications are under version control.
In order to update any one of these documents, a change request should be
raised and approved by management.
A written process is also needed for changing the internal document to match
the latest industry standards or official regulatory documents and/or
pharmacopoeias.
Important laboratory SOPs
The laboratory manual and written procedures should reflect the requirements
of ISO 17025. Even if a laboratory does not intend to be certified to this standard,
there are some essential procedures that are needed, these include:
Laboratory management and organization
Records, documentation and archiving
Reagent and standard quality control
Test method validation and control
Instrument calibration and maintenance programs
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by electronic means are strictly prohibited.
Page 11 of 52