Page 32 - Pharmaceutical Analytical Chemistry II - Pharm D- 02-06-07102
P. 32
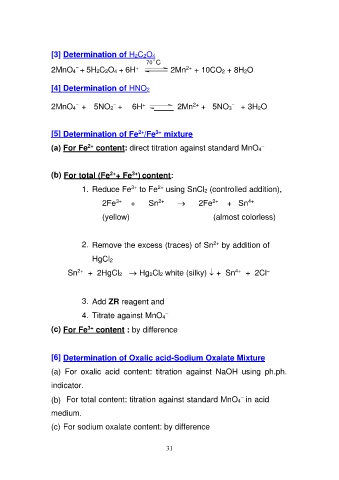
[3] Determination of H2C2O4
70 o C
2MnO4− + 5H2C2O4 + 6H+
2Mn2+ + 10CO2 + 8H2O
[4] Determination of HNO2 2Mn2+ + 5NO3− + 3H2O
2MnO4− + 5NO2− + 6H+
[5] Determination of Fe2+/Fe3+ mixture
(a) For Fe2+ content: direct titration against standard MnO4–
(b) For total (Fe2++ Fe3+) content:
1. Reduce Fe3+ to Fe2+ using SnCl2 (controlled addition),
2Fe3+ + Sn2+ → 2Fe2+ + Sn4+
(yellow) (almost colorless)
2. Remove the excess (traces) of Sn2+ by addition of
HgCl2
Sn2+ + 2HgCl2 → Hg2Cl2 white (silky) + Sn4+ + 2Cl–
3. Add ZR reagent and
4. Titrate against MnO4–
(c) For Fe3+ content : by difference
[6] Determination of Oxalic acid-Sodium Oxalate Mixture
(a) For oxalic acid content: titration against NaOH using ph.ph.
indicator.
(b) For total content: titration against standard MnO4– in acid
medium.
(c) For sodium oxalate content: by difference
31