Page 6 - Pharmaceutical Analytical Chemistry II - Pharm D- 02-06-07102
P. 6
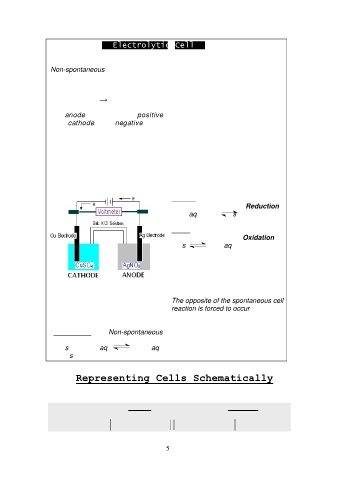
Electrolytic Cell
Non-spontaneous Redox reaction is forced to occur at electrodes
It requires an external source of electrical energy for operation
Electric energy → Chemical change (Redox reaction)
The anode is connected to positive terminal of an external voltage source
while cathode to the negative terminal.
The electrons flow from negative terminal of voltage source (electron rich) to
cathode where reduction occurs,
Current is sustained by oxidation at anode producing electrons that flow to
the positive terminal of voltage source
Cathode Cu metal electrode/ CuSO4
Copper "half-reaction" Reduction
Cu2+ (aq) + 2e- Cu(s)
Anode Ag metal electrode/ AgNO3
Silver "half-reaction" Oxidation
Ag(s) Ag+(aq) + e-
The copper electrode is forced to
become cathode (Reduction) and
the silver electrode is forced to
become anode (Oxidation)
The opposite of the spontaneous cell
reaction is forced to occur
Cell reaction Non-spontaneous
2Ag(s) + Cu2+ (aq) 2Ag+(aq)
+ Cu(s)
Representing Cells Schematically
By convention, Cu/Cu SO4 Cathode
Zn/ZnSO4 Anode
ZnZn2+ (1.0 M) Cu2+ (1.0 M) Cu
5