Page 65 - Pharmaceutical Analytical Chemistry II - Pharm D- 02-06-07102
P. 65
The equilibrium constants K1, K2, Kn are called stepwise stability
constant.
Kst = K1 × K2 × ... Kn
Kst is the stability constant of the complex, or called formation constant
Kf
Knowledge of stability constant values is of considerable
importance in analytical chemistry, since they provide information about
the concentration of the various complexes formed by a metal in specific
equilibrium mixtures. The stability of a complex will obviously be related
to (a) the complexing ability of the metal ion involved, and (b) the
characteristics of the ligand.
Chelate Effect
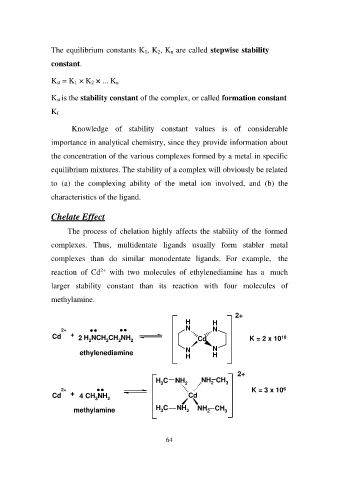
The process of chelation highly affects the stability of the formed
complexes. Thus, multidentate ligands usually form stabler metal
complexes than do similar monodentate ligands. For example, the
reaction of Cd2+ with two molecules of ethylenediamine has a much
larger stability constant than its reaction with four molecules of
methylamine.
2+ HH 2+
NN K = 2 x 1010
Cd + 2 H2NCH2CH2NH2
ethylenediamine Cd
NN
HH
2+ + 4 CH3NH2 H3C NH2 NH2 CH3 2+
Cd K = 3 x 106
Cd
H3C NH2 NH2 CH3
methylamine
64