Page 71 - Pharmaceutical Analytical Chemistry II - Pharm D- 02-06-07102
P. 71
ions. The chelate must have a different color from the free indicator. The
indicator must release the metal ion to the EDTA titrant at pM value very
close to the end point. The metal-indicator complex should be less stable
than metal-EDTA complex otherwise EDTA will not displace it from its
metal complex. On the other hand, it must not be too weak otherwise the
EDTA will start replacing it at the beginning of the titration and a diffuse
end point will result.
Most metal ion indicators are also acid-base indicators. Because
the color of free indicator is pH-dependent, most indicators can be used
only in certain pH ranges at which the color contrast between the free
indicator and the metal-indicator complex can be readily observed.
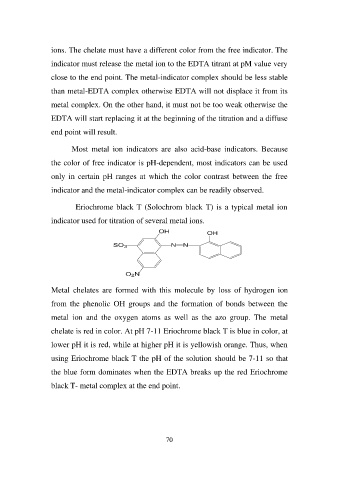
Eriochrome black T (Solochrom black T) is a typical metal ion
indicator used for titration of several metal ions.
OH OH
SO3 NN
O2N
Metal chelates are formed with this molecule by loss of hydrogen ion
from the phenolic OH groups and the formation of bonds between the
metal ion and the oxygen atoms as well as the azo group. The metal
chelate is red in color. At pH 7-11 Eriochrome black T is blue in color, at
lower pH it is red, while at higher pH it is yellowish orange. Thus, when
using Eriochrome black T the pH of the solution should be 7-11 so that
the blue form dominates when the EDTA breaks up the red Eriochrome
black T- metal complex at the end point.
70