Page 79 - Pharmaceutical Analytical Chemistry II - Pharm D- 02-06-07102
P. 79
AgNO3 with another standard solution usually ammonium
thiocyanate (NH4SCN).
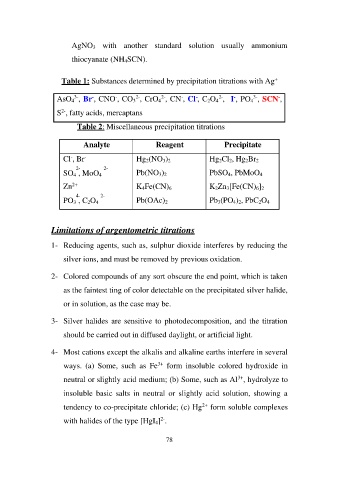
Table 1: Substances determined by precipitation titrations with Ag+
AsO43-, Br-, CNO-, CO32-, CrO42-, CN-, Cl-, C2O42-, I-, PO43-, SCN-,
S2-, fatty acids, mercaptans
Table 2: Miscellaneous precipitation titrations
Analyte Reagent Precipitate
Hg2(NO3)2 Hg2Cl2, Hg2Br2
Cl-, Br- Pb(NO3)2 PbSO4, PbMoO4
K4Fe(CN)6 K2Zn3[Fe(CN)6]2
2- 2- Pb(OAc)2 Pb3(PO4)2, PbC2O4
SO4 , MoO4
Zn2+
4- 2-
PO3 , C2O4
Limitations of argentometric titrations
1- Reducing agents, such as, sulphur dioxide interferes by reducing the
silver ions, and must be removed by previous oxidation.
2- Colored compounds of any sort obscure the end point, which is taken
as the faintest ting of color detectable on the precipitated silver halide,
or in solution, as the case may be.
3- Silver halides are sensitive to photodecomposition, and the titration
should be carried out in diffused daylight, or artificial light.
4- Most cations except the alkalis and alkaline earths interfere in several
ways. (a) Some, such as Fe3+ form insoluble colored hydroxide in
neutral or slightly acid medium; (b) Some, such as Al3+, hydrolyze to
insoluble basic salts in neutral or slightly acid solution, showing a
tendency to co-precipitate chloride; (c) Hg2+ form soluble complexes
with halides of the type [HgI4]2-.
78