Page 87 - Pharmaceutical Analytical Chemistry II - Pharm D- 02-06-07102
P. 87
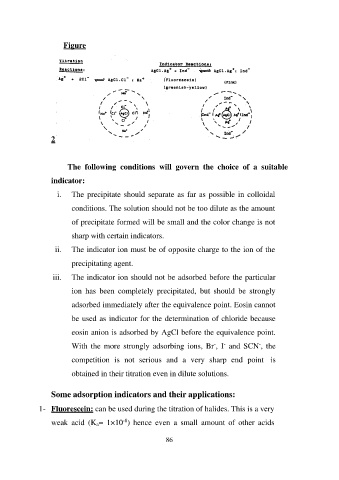
Figure
2
The following conditions will govern the choice of a suitable
indicator:
i. The precipitate should separate as far as possible in colloidal
conditions. The solution should not be too dilute as the amount
of precipitate formed will be small and the color change is not
sharp with certain indicators.
ii. The indicator ion must be of opposite charge to the ion of the
precipitating agent.
iii. The indicator ion should not be adsorbed before the particular
ion has been completely precipitated, but should be strongly
adsorbed immediately after the equivalence point. Eosin cannot
be used as indicator for the determination of chloride because
eosin anion is adsorbed by AgCl before the equivalence point.
With the more strongly adsorbing ions, Br-, I- and SCN-, the
competition is not serious and a very sharp end point is
obtained in their titration even in dilute solutions.
Some adsorption indicators and their applications:
1- Fluorescein: can be used during the titration of halides. This is a very
weak acid (Ka= 1×10-8) hence even a small amount of other acids
86