Page 241 - Applied Pharmacognosy
P. 241
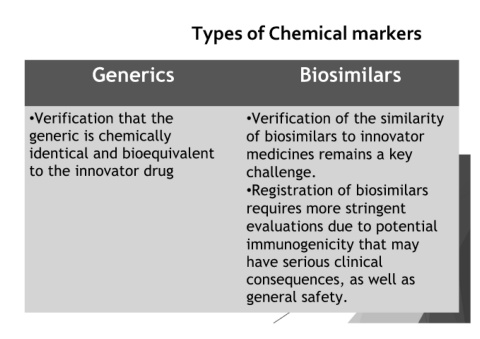
Types of Chemical markers
Generics Biosimilars
•Verification that the •Verification of the similarity
generic is chemically of biosimilars to innovator
identical and bioequivalent medicines remains a key
to the innovator drug challenge.
•Registration of biosimilars
requires more stringent
evaluations due to potential
immunogenicity that may
have serious clinical
consequences, as well as
general safety.