Page 5 - Analytical Chemistry 1
P. 5
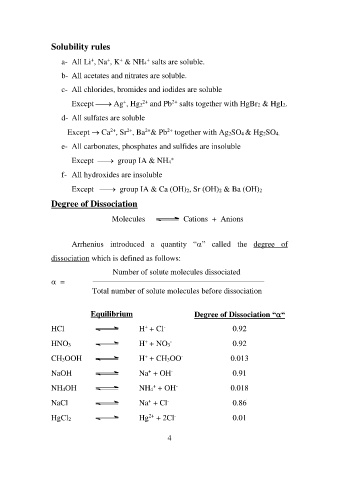
Solubility rules
a- All Li+, Na+, K+ & NH4+ salts are soluble.
b- All acetates and nitrates are soluble.
c- All chlorides, bromides and iodides are soluble
Except ⎯→ Ag+, Hg22+ and Pb2+ salts together with HgBr2 & HgI2.
d- All sulfates are soluble
Except → Ca2+, Sr2+, Ba2+& Pb2+ together with Ag2SO4 & Hg2SO4.
e- All carbonates, phosphates and sulfides are insoluble
Except ⎯→ group IA & NH4+
f- All hydroxides are insoluble
Except ⎯→ group IA & Ca (OH)2, Sr (OH)2 & Ba (OH)2
Degree of Dissociation
Molecules Cations + Anions
Arrhenius introduced a quantity “” called the degree of
dissociation which is defined as follows:
= Number of solute molecules dissociated
___________________________________________________________________
Total number of solute molecules before dissociation
Equilibrium Degree of Dissociation ““
0.92
HCl H+ + Cl- 0.92
0.013
HNO3 H+ + NO3- 0.91
0.018
CH3OOH H+ + CH3OO- 0.86
0.01
NaOH Na+ + OH-
NH4OH NH4+ + OH-
NaCl Na+ + Cl-
HgCl2 Hg2+ + 2Cl-
4