Page 86 - Pharmd general phytochemistry I-Final2024_LEUCTERS
P. 86
Properties
Solubility:
• they are usually soluble in water, aqueous alcohol, but almost
insoluble in fat solvents (petroleum ether)
• As a rule, the higher the number of the sugar units, the
greater the solubility in water, but the lower the solubility in
chloroform.
Stability:
1- Effect of acids:
• - Treatment of cardiac glycosides with dilute acids result in
hydrolysis of glycosidic bonds and liberation of the aglycones
and the individual sugar units.
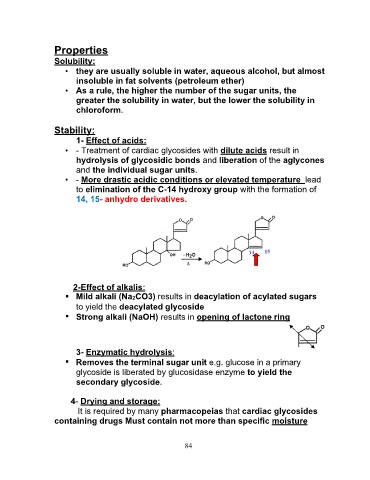
• - More drastic acidic conditions or elevated temperature lead
to elimination of the C-14 hydroxy group with the formation of
14, 15- anhydro derivatives.
2-Effect of alkalis:
Mild alkali (Na2CO3) results in deacylation of acylated sugars
to yield the deacylated glycoside
Strong alkali (NaOH) results in opening of lactone ring
3- Enzymatic hydrolysis:
Removes the terminal sugar unit e.g. glucose in a primary
glycoside is liberated by glucosidase enzyme to yield the
secondary glycoside.
4- Drying and storage:
It is required by many pharmacopeias that cardiac glycosides
containing drugs Must contain not more than specific moisture
84