Page 81 - Pharmaceutical Analytical Chemistry II - Pharm D Clinical- 07-PA202
P. 81
shielding of the dissociated ionic species, for example BaSO4 in presence
of NaNO3 (diverse ion).
Fractional precipitation
It is the process of successive precipitation of salts having different
solubilities using one precipitating agent. A good representative example
is the successive precipitation of iodide and chloride ions using silver ion
as precipitating agent (Ksp of AgI = 8.3×10-17 and of AgCl = 1.1×10-10).
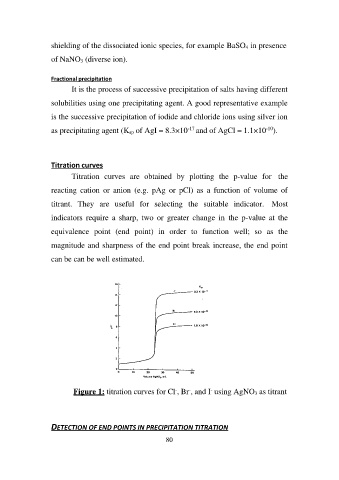
Titration curves
Titration curves are obtained by plotting the p-value for the
reacting cation or anion (e.g. pAg or pCl) as a function of volume of
titrant. They are useful for selecting the suitable indicator. Most
indicators require a sharp, two or greater change in the p-value at the
equivalence point (end point) in order to function well; so as the
magnitude and sharpness of the end point break increase, the end point
can be can be well estimated.
Figure 1: titration curves for Cl-, Br-, and I- using AgNO3 as titrant
DETECTION OF END POINTS IN PRECIPITATION TITRATION
80