Page 87 - Pharmaceutical Analytical Chemistry II - Pharm D Clinical- 07-PA202
P. 87
reduces the already minute ionization, thus rendering the detection of
the end point (which depends essentially upon the adsorption of the
free anion) either impossible or difficult to observe. The optimum pH
range is between 7 and 10.
2- Dichlorofluorescein: is a stronger acid and may be utilized in
slightly acid solution of pH greater than 4.4. This indicator has the
further advantage that it is applicable in very dilute solutions.
3- Eosin (tetrabromofluorescein): is a stronger acid and can be used
down to a pH of 1.2. The color change is sharper in an acetic acid
solution.
4- Rhodamine 6G: The hydrochloride of a basic dye, is a good
indicator for the titration of silver ions with a standard bromide
solution in dilute nitric acid solution. As long as silver ions are
present in excess, the indicator cation is not noticeably adsorbed by
silver bromide. At the equivalence point or after a very slight excess
of bromide has been added, the precipitate adsorbs the dye strongly
and acquires a blue violet color.
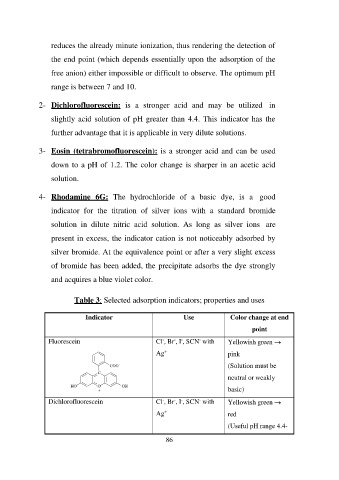
Table 3: Selected adsorption indicators; properties and uses
Indicator Use Color change at end
Cl-, Br-, I-, SCN- with point
Fluorescein Ag+
Yellowish green →
COO- Cl-, Br-, I-, SCN- with pink
C Ag+ (Solution must be
neutral or weakly
HO O OH 86 basic)
+ Yellowish green →
red
Dichlorofluorescein (Useful pH range 4.4-