Page 108 - eBook Version 8 Book 1 of 2 JUL 2022
P. 108
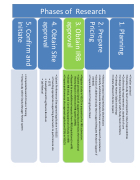
Phases of Research
1. Planning
• Finalize protocol
• Develop study team and respective roles/responsibilities
• Outline study requirements vs. standard of care
• Undergo Credentialing of staff /study team members
• Undergo Human Subjects trainings
• Initiate eResearch Access request
2. Prepare Pricing
• Review protocol required study observations
3. Obtain IRB approval
• Register study in VELOS in preparation for IRB submission.
4. Obtain Site approval
• Submit Performance Site approval application in VELOS
• Providing appropriate supporting documents to performance site
5. Confirm and initiate
• Complete Research Finance Training
• Setup study within Study Manager Reveal system
• Identify and Delineate Standard of Care versus Research procedures
• Decision of additional study team support (Request Research Support if needed)
• Prepare Research Pricing Quote Sheet
• Submit protocol (and all supporting documents) to the Institutional Review Board in electronic IRB system (eIRB)
• Respond to any additional requests from IRB (stipulations) in eIRB.
• Obtain IRB initial, and Continuing approval of study in eIRB
• Prepare performance site approval application (PSA) in VELOS
• 1572
• Investigational Drug/Device Brochure • Lab Manual