Page 269 - eBook Version 8 Book 1 of 2 JUL 2022
P. 269
EPIC ResearchBilling
Research Administration- Effective Fall 2018 QRG v.4.0
Research Charges Entry
(6b. Coordination Charges cont.)
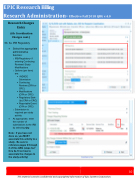
6b. 3a. IRB Regulatory
• Select the appropriate administrative
function:
o IRB/Regulatory- if
entering Continuing Reviews/ Study Modifications
o Options (per item) are:
▪ IND/IDE Submission
▪ Continuing Reviews (CRA or CRC)
▪ Modifications (CRA or CRC)
▪ Regulatory Start- Up (CRA or CRC)
▪ Reportable Event (CRA or CRC)
o Submission is required per study activity
• As appropriate, enter the number of
submissions to the IRB by selecting Qty.
Note: If you have not done so, you MUST associate this activity to a study. Be sure to reference pages 6 through 8 of this QRG (steps 6a.7 thru 6a.10 on how to associate the charges to the study activity)
10
This material contains confidential and copyrighted information of Epic Systems Corporation.