Page 96 - Inventory Report
P. 96
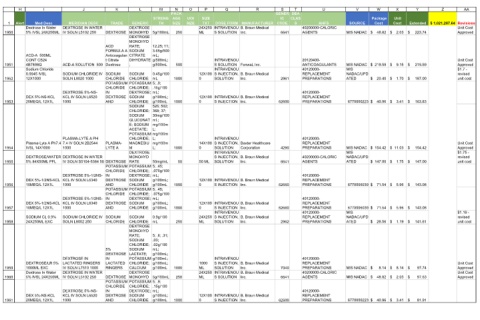
Revisions Unit Cost Approved Unit Cost Approved $1.7 - revised unit cost Unit Cost Approved $1.75 - revised unit cost $1.19 - revised unit cost Unit Cost Approved Unit Cost Approved
AA
Z $ 1,021,287.64
Extended
Y $ 223.74 $ 219.59 $ 187.00 $ 163.83 $ 154.42 $ 147.00 $ 143.08 $ 143.08 $ 141.61 $ 97.74 $ 97.63 $ 81.91
Unit
X Cost $ 2.03 $ 9.15 $ 1.70 $ 3.41 $ 11.03 $ 1.75 $ 5.96 $ 5.96 $ 1.19 $ 8.14 $ 2.03 $ 3.41
Package Cost
W $ 48.82 $ 219.59 $ 20.40 $ 40.96 $ 154.42 $ 147.00 $ 71.54 $ 71.54 $ 28.56 $ 8.14 $ 48.82 $ 40.96
V SOURCE MIS NADAC MIS NADAC MIS NADAC/UPD 6770000223 MIS NADAC/UPD ATED 6770006039 6770006039 MIS NADAC/UPD MIS NADAC 6770000223
40200000-CALORIC 20120400- ANTICOAGULANTS 40120000- REPLACEMENT 40120000- REPLACEMENT 40120000- REPLACEMENT 40200000-CALORIC 40120000- REPLACEMENT 40120000- REPLACEMENT 40120000- REPLACEMENT 40120000- REPLACEMENT 40200000-CALORIC 40120000- REPLACEMENT
U AHFS AGENTS PREPARATIONS ATED PREPARATIONS PREPARATIONS MIS NADAC AGENTS PREPARATIONS PREPARATIONS PREPARATIONS ATED PREPARATIONS MIS NADAC AGENTS PREPARATIONS
DEA
T CLAS S
S GENER IC CODE 6641 2961 62600 4290 6641 62660 62660 2962 7040 6641 62600
MANUFACTURER
R B. Braun Medical Inc. Fenwal, Inc. B. Braun Medical Inc. B. Braun Medical Inc. Baxter Healthcare Corporation B. Braun Medical Inc. B. Braun Medical Inc. B. Braun Medical Inc. B. Braun Medical Inc. B. Braun Medical Inc. B. Braun Medical Inc. B. Braun Medical Inc.
DOSE FORM S INJECTION, S INJECTION, S INJECTION, S INJECTION, S INJECTION,
Q INTRAVENOU S SOLUTION INTRAVENOU S SOLUTION INTRAVENOU SOLUTION INTRAVENOU S INJECTION INTRAVENOU SOLUTION INTRAVENOU SOLUTION INTRAVENOU S INJECTION INTRAVENOU S INJECTION INTRAVENOU SOLUTION INTRAVENOU SOLUTION INTRAVENOU S SOLUTION INTRAVENOU S INJECTION
SIZE
P TXT 24X250 ML 12X100 0 12X100 0 14X100 0 50 ML 12X100 0 12X100 0 24X250 ML 1000 ML 24X250 ML 12X100 0
UOI
O SIZE
PACK AGE 250 500 1000 1000 1000 50 1000 1000 250 1000 250 1000
N SIZE
M STRENG TH 5g/100mL 12.25; 11; 3.65g/500 mL; g/500mL; g/500mL 0.45g/100 mL .15g/100 mL; g/100mL; g/100mL 526; 502; 368; 37; 30mg/100 mL; mg/100m L; L; mg/100m L; 50mg/mL .075g/100 mL; g/100mL; g/100mL .075g/100 mL; g/100mL; g/100mL 0.9g/100 mL 5; .6; .31; .03; .02g/100 mL; g/100mL; g/100mL; g/100mL 5g/100mL .15g/100 mL; g/100mL; g/100mL
L GENERIC DEXTROSE MONOHYD DEXTROSE MONOHYD RATE; SODIUM CITRATE DIHYDRATE ; SODIUM CHLORIDE POTASSIUM POTASSIUM 5; .9; CHLORIDE; DEXTROSE; SODIUM CHLORIDE SODIUM CHLORIDE; SODIUM GLUCONAT E; SODIUM ACETATE; POTASSIUM mg/100m CHLORIDE; MAGNESIU M DEXTROSE MONOHYD RATE POTASSIUM POTASSIUM 5; .45; CHLORIDE; DEXTROSE; SODIUM CHLORIDE POTASSIUM POTASSIUM 5; .45; CHLORIDE; DEXTROSE; SODIUM CHLORIDE SODIUM CHLORIDE DEXTROSE MONOHYD RATE; SODIUM CHLORIDE; SODIUM LACTATE; POTASSIUM g/100mL; CHLORIDE; CALCIUM DEXTROSE MONOHYD POTASSIUM POTASSIUM 5; .9; CHLORIDE; DEXTROSE; SODIUM
K TRADE DEXTROSE ACD FORMULA A Anticoagulan t Citrate Dextrose SODIUM CHLORIDE CHLORIDE IN DEXTROSE AND PLASMA- LYTE A DEXTROSE CHLORIDE IN DEXTROSE AND CHLORIDE IN DEXTROSE AND SODIUM CHLORIDE 5% DEXTROSE IN LACTATED RINGERS DEXTROSE CHLORIDE IN DEXTROSE AND
J MERIDIAN DESC DEXTROSE IN WATER IV SOLN L5102 250 ACD-A SOLUTION 500 SODIUM CHLORIDE IV SOLN L8020 1000 DEXTROSE 5%-NS- KCL IV SOLN L6520 1000 PLASMA-LYTE A PH 1000 DEXTROSE/WATER DEXTROSE IN WATER IV SOLN S5104-5384 50 DEXTROSE 5%-1/2NS- KCL IV SOLN L6340 1000 DEXTROSE 5%-1/2NS- KCL IV SOLN L6340 1000 SODIUM CHLORIDE IV SOLN L8002 250 DEXTROSE IN LACTATED RINGERS IV SOLN L7510 1000 DEXTROSE IN WATER IV SOLN L5102 250 DEXTROSE 5%-NS- KCL IV SOLN L6520 1000
Med Desc Dextrose In Water 5% IVSL 24X250ML ACD-A 500ML CONT CS24 4B7898Q Sodium Chloride 0.0045 IVSL 12X1000 DEX 5%-NS-KCL 20MEQ/L 12X1L IVSL 14X1000 5% 84X50ML PFL DEX 5%-1/2NS-KCL 10MEQ/L 12X1L DEX 5%-1/2NS-KCL 10MEQ/L 12X1L SODIUM CL 0.9% 24X250ML EXC DEXTROSE/LR 5% 1000ML EXC Dextrose In Water 5% IVSL 24X250ML DEX 5%-NS-KCL 20MEQ/L 12X1L
I Plasma-Lyte A Ph7.4 7.4 IV SOLN 2B2544
Alert
H
1 1950 1951 1952 1953 1954 1955 1956 1957 1958 1959 1960 1961