Page 56 - Lab Manual & Project class 12
P. 56
LABORATORY MANUAL CHEMISTRY
(b) Acetate gives deep red colour on reaction with neutral ferric chloride
solution due to the formation of complex ion which decomposes on
heating to give Iron (III) dihydroxyacetate as brown red precipitate.
–
+
3+
6 CH COO + 3Fe + 2H O [Fe (OH) (CH COO) ] + 2H +
3 2 3 2 3 6
+
[Fe (OH) (CH COO) ] + 4H O 3[Fe (OH) (CH COO)] + 3CH COOH + H +
6
2
3
3
2
3
2
3
Iron(III)dihydroxyacetate
(Brown-red precipitate)
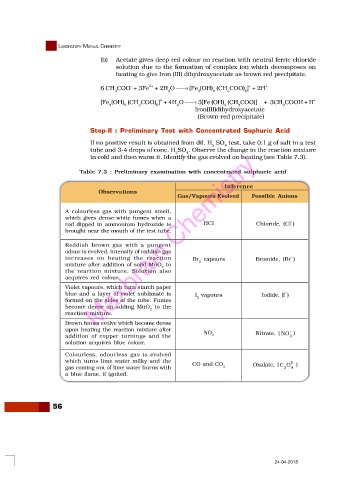
Step-II : Preliminary Test with Concentrated Suphuric Acid
If no positive result is obtained from dil. H SO test, take 0.1 g of salt in a test
4
2
tube and 3-4 drops of conc. H SO . Observe the change in the reaction mixture
2
4
Maxbrain Chemistry
in cold and then warm it. Identify the gas evolved on heating (see Table 7.3).
Table 7.3 : Preliminary examination with concentrated sulphuric acid
Inference
Observations
Gas/Vapours Evolved Possible Anions
A colourless gas with pungent smell,
which gives dense white fumes when a
–
rod dipped in ammonium hydroxide is HCl Chloride, (Cl )
brought near the mouth of the test tube.
Reddish brown gas with a pungent
odour is evolved. Intensity of reddish gas
–
increases on heating the reaction Br vapours Bromide, (Br )
mixture after addition of solid MnO to 2
2
the reaction mixture. Solution also
acquires red colour.
Violet vapours, which turn starch paper
blue and a layer of violet sublimate is I vapours Iodide, (I )
–
formed on the sides of the tube. Fumes 2
become dense on adding MnO to the
2
reaction mixture.
Brown fumes evolve which become dense
upon heating the reaction mixture after NO –
addition of copper turnings and the 2 Nitrate, ( NO )
3
solution acquires blue colour.
Colourless, odourless gas is evolved
which turns lime water milky and the CO and CO 2–
gas coming out of lime water burns with 2 Oxalate, ( C O 4 )
2
a blue flame, if ignited.
56
24-04-2018