Page 84 - Lab Manual & Project class 12
P. 84
LABORATORY MANUAL CHEMISTRY
SPECIMEN RECORD OF SALT ANALYSIS
Aim
To analyse the given salt for one anion and one cation present in it.
Material required
• Boiling tubes, test tubes, test tube holder, test tube stand, delivery tube, corks,
filter papers, reagents
Maxbrain Chemistry
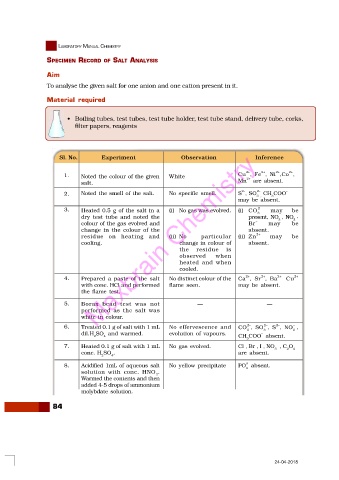
Sl. No. Experiment Observation Inference
2+
2+
2+
2+
1. Noted the colour of the given White Cu , Fe , Ni ,Co ,
2+
salt. Mn are absent.
2–
2–
2. Noted the smell of the salt. No specific smell. S , SO CH COO –
3 ,
3
may be absent.
2–
3. Heated 0.5 g of the salt in a (i) No gas was evolved. (i) CO may be
3
–
dry test tube and noted the present, NO , NO 2 – ,
3
–
colour of the gas evolved and Br may be
change in the colour of the absent.
2+
residue on heating and (ii) No particular (ii) Zn may be
cooling. change in colour of absent.
the residue is
observed when
heated and when
cooled.
2+
2+
4. Prepared a paste of the salt No distinct colour of the Ca , Sr , Ba 2+ Cu 2+
with conc. HCl and performed flame seen. may be absent.
the flame test.
5. Borax bead test was not — —
performed as the salt was
white in colour.
6. Treated 0.1 g of salt with 1 mL No effervescence and CO , SO , S , NO ,
2–
2–
–
2–
3
2
3
dil.H SO and warmed. evolution of vapours. –
2 4 CH COO absent.
3
–
–
–
–
7. Heated 0.1 g of salt with 1 mL No gas evolved. Cl , Br , I , NO , C O 4 –
2
3
conc. H SO . are absent.
2 4
3–
8. Acidified 1mL of aqueous salt No yellow precipitate PO absent.
4
solution with conc. HNO .
3
Warmed the contents and then
added 4-5 drops of ammonium
molybdate solution.
84
24-04-2018