Page 90 - Lab Manual & Project class 12
P. 90
C. Lucas test
Take 1 mL of compound in a test tube. Add 10 mL of Lucas reagent.
Shake well and note the time for the separation of two distinct layers.
Note : Lucas test is applicable to only those alcohols which are soluble
in the reagent because the test is based on separation of alkyl
halides as separate layer.
Maxbrain Chemistry
The –OH group attached directly to the ring carbon of an aromatic
ring is called phenolic –OH group. Phenols are weakly acidic,
therefore they are soluble in NaOH solution but at the same time
they are not sufficiently acidic to be soluble is sodium
hydrogencarbonate solution. Phenols give coloured complex with
neutral ferric chloride solution. For example, phenol gives a
complex of violet colour as follows :
3–
6C H OH + FeCl → [Fe(C H O) ] + 3HCl + 3H +
6 5 3 6 5 6
Violet complex
Resorcinol, o–, m– and p–cresol give violet or blue colouration,
catechol gives green colour which rapidly darkens. 1 and 2–Naphthol
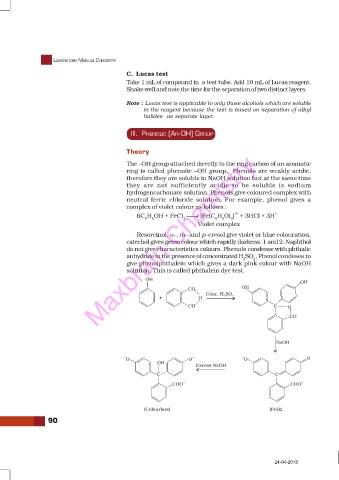
do not give characteristics colours. Phenols condense with phthalic
anhydride in the presence of concentrated HSO , Phenol condeses to
2 4
give phenolphthalein which gives a dark pink colour with NaOH
solution. This is called phthalein dye test.
24-04-2018