Page 36 - MRS Abstracts March 2023
P. 36
were generated. All statistical analysis and data visualisations were done using R studio (v 2023).
Results:
We had 230 patients on Baricitinib and 45 on Tofacitinib for RA. Majority were females (78.5%) and the mean age was 59.4 ± 12.2 years. The mean baseline Disease Activity Score 28 (DAS28) was 5.9 ± 0.9, and a majority of patients were seropositive for rheumatoid factor (56.1%) and/or anti-cyclic citrullinated peptide antibodies (70.5%).
Concomitant medications included methotrexate (43.5%), hydroxychloroquine (18.5%), and steroids (10.3%). The mean baseline weight was 76.2 ± 20.1 kg. Weight at baseline and 9-12 months were available for 157 patients.
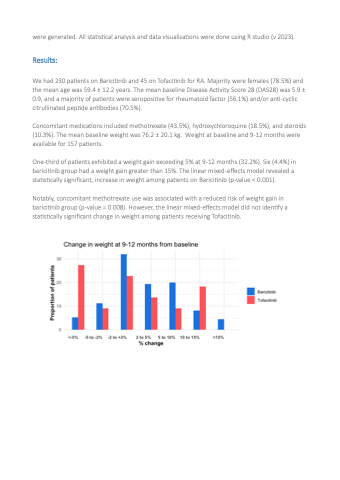
One-third of patients exhibited a weight gain exceeding 5% at 9-12 months (32.2%). Six (4.4%) in baricitinib group had a weight gain greater than 15%. The linear mixed-effects model revealed a statistically significant, increase in weight among patients on Baricitinib (p-value < 0.001).
Notably, concomitant methotrexate use was associated with a reduced risk of weight gain in baricitinib group (p-value = 0.008). However, the linear mixed-effects model did not identify a statistically significant change in weight among patients receiving Tofacitinib.