Page 11 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1 2024-2025
P. 11
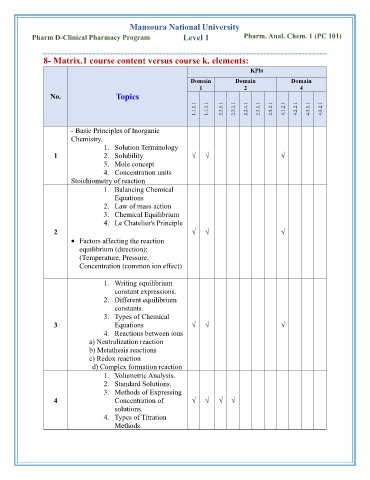
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
8- Matrix.1 course content versus course k. elements:
KPIs
Domain Domain Domain
1 2 4
No. Topics
1.1.1.1 1.1.3.1 2.2.1.1 2.2.3.1 2.2.4.1 2.3.1.1 2.3.2.1 4.1.2.1 4.2.2.1 4.3.1.1 4.3.2.1
- Basic Principles of Inorganic
Chemistry.
1. Solution Terminology
1 2. Solubility √ √ √
3. Mole concept
4. Concentration units
Stoichiometry of reaction
1. Balancing Chemical
Equations
2. Law of mass action
3. Chemical Equilibrium
4. Le Chatelier's Principle
2 √ √ √
• Factors affecting the reaction
equilibrium (direction):
(Temperature, Pressure,
Concentration (common ion effect)
1. Writing equilibrium
constant expressions.
2. Different equilibrium
constants.
3. Types of Chemical
3 Equations √ √ √
4. Reactions between ions
a) Neutralization reaction
b) Metathesis reactions
c) Redox reaction
d) Complex formation reaction
1. Volumetric Analysis.
2. Standard Solutions.
3. Methods of Expressing
4 Concentration of √ √ √ √
solutions.
4. Types of Titration
Methods.