Page 56 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 56
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
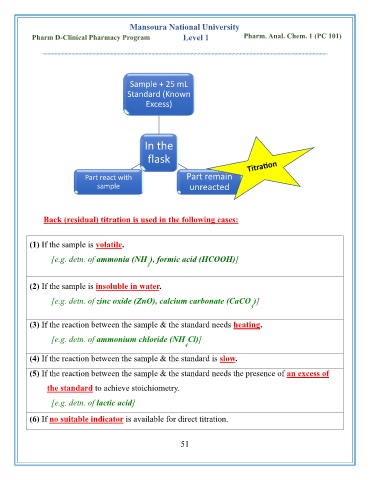
Sample + 25 mL
Standard (Known
Excess)
In the
flask
Part react with Part remain
sample unreacted
Back (residual) titration is used in the following cases:
(1) If the sample is volatile.
[e.g. detn. of ammonia (NH ), formic acid (HCOOH)]
3
(2) If the sample is insoluble in water.
[e.g. detn. of zinc oxide (ZnO), calcium carbonate (CaCO )]
3
(3) If the reaction between the sample & the standard needs heating.
[e.g. detn. of ammonium chloride (NH Cl)]
4
(4) If the reaction between the sample & the standard is slow.
(5) If the reaction between the sample & the standard needs the presence of an excess of
the standard to achieve stoichiometry.
[e.g. detn. of lactic acid]
(6) If no suitable indicator is available for direct titration.
51