Page 8 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 8
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
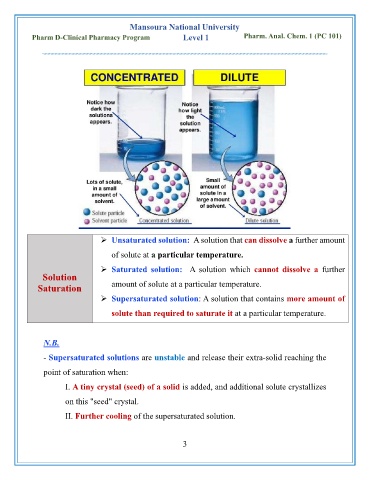
➢ Unsaturated solution: A solution that can dissolve a further amount
of solute at a particular temperature.
➢ Saturated solution: A solution which cannot dissolve a further
Solution
Saturation amount of solute at a particular temperature.
➢ Supersaturated solution: A solution that contains more amount of
solute than required to saturate it at a particular temperature.
N.B.
- Supersaturated solutions are unstable and release their extra-solid reaching the
point of saturation when:
I. A tiny crystal (seed) of a solid is added, and additional solute crystallizes
on this "seed" crystal.
II. Further cooling of the supersaturated solution.
3