Page 136 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 136
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
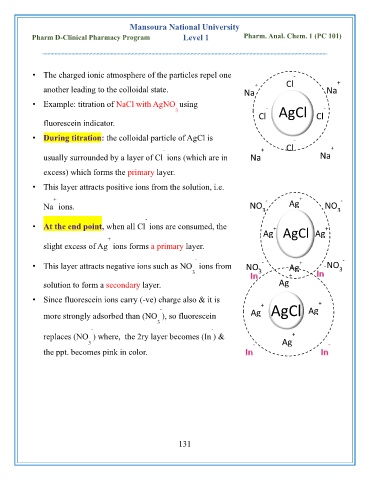
• The charged ionic atmosphere of the particles repel one -
+ Cl +
another leading to the colloidal state. Na Na
• Example: titration of NaCl with AgNO using
3 - AgCl -
Cl Cl
fluorescein indicator.
• During titration: the colloidal particle of AgCl is -
- + Cl +
usually surrounded by a layer of Cl ions (which are in Na Na
excess) which forms the primary layer.
• This layer attracts positive ions from the solution, i.e.
+ - + -
Na ions. NO Ag NO
3
3
-
• At the end point, when all Cl ions are consumed, the + +
Ag AgCl Ag
+
slight excess of Ag ions forms a primary layer.
- -
-
+
• This layer attracts negative ions such as NO ions from NO Ag NO
3 3 3
+
solution to form a secondary layer. Ag
• Since fluorescein ions carry (-ve) charge also & it is +
- + AgCl Ag
more strongly adsorbed than (NO ), so fluorescein Ag
3
- -
replaces (NO ) where, the 2ry layer becomes (In ) & +
3 Ag
the ppt. becomes pink in color.
131