Page 354 - Physics Coursebook 2015 (A level)
P. 354
Cambridge International A Level Physics
8 a A 500 W kettle contains 300 g of water at 20 °C. Calculate the minimum time it would take to raise the
temperature of the water to boiling point. [5]
b The kettle is allowed to boil for 2 minutes. Calculate the mass of water that remains in the kettle.
State any assumptions that you make. [4]
(Specific heat capacity of water = 4.18 × 103 J kg−1 °C−1,
specific latent heat of vaporisation of water = 2.26 × 106 J kg−1.)
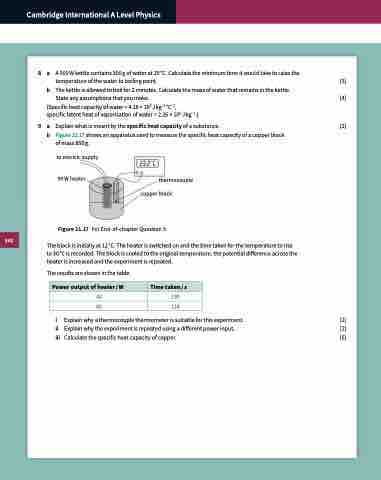
9 a Explain what is meant by the specific heat capacity of a substance. [2] b Figure 21.17 shows an apparatus used to measure the specific heat capacity of a copper block
of mass 850 g.
to electric supply 90 W heater
thermocouple copper block
Figure 21.17 For End-of-chapter Question 9.
The block is initially at 12 °C. The heater is switched on and the time taken for the temperature to rise to 30 °C is recorded. The block is cooled to the original temperature, the potential difference across the heater is increased and the experiment is repeated.
The results are shown in the table.
40 190
60 114
i Explain why a thermocouple thermometer is suitable for this experiment. [2]
ii Explain why the experiment is repeated using a different power input. [2]
iii Calculatethespecificheatcapacityofcopper. [5]
Power output of heater / W
Time taken / s
342