Page 500 - Physics Coursebook 2015 (A level)
P. 500
Cambridge International A Level Physics
12
a b
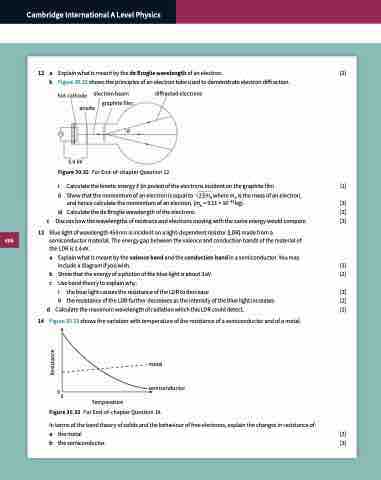
Explain what is meant by the de Broglie wavelength of an electron. [2] Figure 30.32 shows the principles of an electron tube used to demonstrate electron diffraction.
hot cathode anode
5.0 kV
electron beam
graphite film
θ
diffracted electrons
13
Blue light of wavelength 450 nm is incident on a light-dependent resistor (LDR) made from a semiconductor material. The energy gap between the valence and conduction bands of the material of the LDR is 2.4 eV.
a Explain what is meant by the valence band and the conduction band in a semiconductor. You may
include a diagram if you wish. [3]
b Show that the energy of a photon of the blue light is about 3 eV. [2]
c Use band theory to explain why:
i the blue light causes the resistance of the LDR to decrease [3]
ii the resistance of the LDR further decreases as the intensity of the blue light increases. [2]
d Calculate the maximum wavelength of radiation which this LDR could detect. [2]
Figure 30.33 shows the variation with temperature of the resistance of a semiconductor and of a metal.
14
c
Figure 30.32 For End-of-chapter Question 12.
i Calculate the kinetic energy E (in joules) of the electrons incident on the graphite film. [1]
ii Show that the momentum of an electron is equal to 2 Eme where me is the mass of an electron,
and hence calculate the momentum of an electron. (me = 9.11 × 10–31 kg). [3]
iii CalculatethedeBrogliewavelengthoftheelectrons. [2] Discuss how the wavelengths of neutrons and electrons moving with the same energy would compare. [3]
00
Temperature
metal
semiconductor
Figure 30.33 For End-of-chapter Question 14.
In terms of the band theory of solids and the behaviour of free electrons, explain the changes in resistance of:
a the metal [3]
b the semiconductor. [3]
488
Resistance