Page 224 - Geosystems An Introduction to Physical Geography 4th Canadian Edition
P. 224
188 part II The Water, Weather, and Climate Systems
Midnight
35° 30° 25° 20° 15° 10°
5°
0° –5°
–10°
100
80
60
40
20
0
July 22 July 23 66
60 55 50 45
40
35
30
25
20
15
10
5
Noon P.M. Midnight
Highest temperature
Noon P.M. Midnight
Lowest relative humidity
Lowest temperature
Highest relative humidity
e
–40–30–20–10 0 10 20 30 40 Temperature (°C)
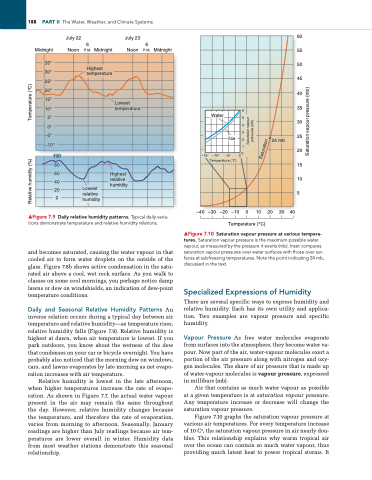
▲Figure 7.9 Daily relative humidity patterns. Typical daily varia- tions demonstrate temperature and relative humidity relations.
and becomes saturated, causing the water vapour in that cooled air to form water droplets on the outside of the glass. Figure 7.8b shows active condensation in the satu rated air above a cool, wet rock surface. As you walk to classes on some cool mornings, you perhaps notice damp lawns or dew on windshields, an indication of dewpoint temperature conditions.
Daily and Seasonal Relative Humidity Patterns An inverse relation occurs during a typical day between air temperature and relative humidity—as temperature rises, relative humidity falls (Figure 7.9). Relative humidity is highest at dawn, when air temperature is lowest. If you park outdoors, you know about the wetness of the dew that condenses on your car or bicycle overnight. You have probably also noticed that the morning dew on windows, cars, and lawns evaporates by late morning as net evapo ration increases with air temperature.
Relative humidity is lowest in the late afternoon, when higher temperatures increase the rate of evapo ration. As shown in Figure 7.7, the actual water vapour present in the air may remain the same throughout the day. However, relative humidity changes because the temperature, and therefore the rate of evaporation, varies from morning to afternoon. Seasonally, January readings are higher than July readings because air tem peratures are lower overall in winter. Humidity data from most weather stations demonstrate this seasonal relationship.
▲Figure 7.10 Saturation vapour pressure at various tempera- tures. Saturation vapour pressure is the maximum possible water vapour, as measured by the pressure it exerts (mb). inset compares saturation vapour pressures over water surfaces with those over sur- faces at subfreezing temperatures. Note the point indicating 24 mb, discussed in the text.
Specialized Expressions of Humidity
There are several specific ways to express humidity and relative humidity. Each has its own utility and applica tion. Two examples are vapour pressure and specific humidity.
Vapour Pressure As free water molecules evaporate from surfaces into the atmosphere, they become water va pour. Now part of the air, watervapour molecules exert a portion of the air pressure along with nitrogen and oxy gen molecules. The share of air pressure that is made up of watervapour molecules is vapour pressure, expressed in millibars (mb).
Air that contains as much water vapour as possible at a given temperature is at saturation vapour pressure. Any temperature increase or decrease will change the saturation vapour pressure.
Figure 7.10 graphs the saturation vapour pressure at various air temperatures. For every temperature increase of 10 C°, the saturation vapour pressure in air nearly dou bles. This relationship explains why warm tropical air over the ocean can contain so much water vapour, thus providing much latent heat to power tropical storms. It
6 5 4 3 2 1
–15° –10° –5° 00 Temperature (°C)
Water
Ic
24 mb
n
o
i
t
a
r
u
t
a
S
Relative humidity (%) Temperature (°C)
Saturation vapour pressure (mb)
Saturation vapour pressure (mb)