Page 225 - Geosystems An Introduction to Physical Geography 4th Canadian Edition
P. 225
Chapter 7 Water and Atmospheric Moisture 189
also explains why cold air is “dry” and why cold air to ward the poles does not produce a lot of precipitation (it contains too little water vapour, even though it is near the dewpoint temperature).
As marked on the graph, air at 20°C has a saturation vapour pressure of 24 mb; that is, the air is saturated if the watervapour portion of the air pressure also is at 24 mb. Thus, if the water vapour actually present is exerting a vapour pressure of only 12 mb in 20°C air, the relative humidity is 50% (12 mb 4 24 mb 5 0.50 3 100 5 50%). The inset in Figure 7.10 compares saturation vapour pressure over water and over ice surfaces at subfreezing temperatures. You can see that saturation vapour pressure is greater above a water surface than over an ice surface— that is, it takes more watervapour molecules to saturate air above water than it does above ice. This fact is impor tant to condensation processes and raindroplet forma tion, both of which are described later in this chapter.
Specific Humidity A useful humidity measure is one that remains constant as temperature and pressure change. Specific humidity is the mass of water vapour (in grams) per mass of air (in kilograms) at any specified temperature. Because it is measured in mass, specific hu midity is not affected by changes in temperature or pres sure, as occur when air rises to higher elevations. Spe cific humidity stays constant despite volume changes.*
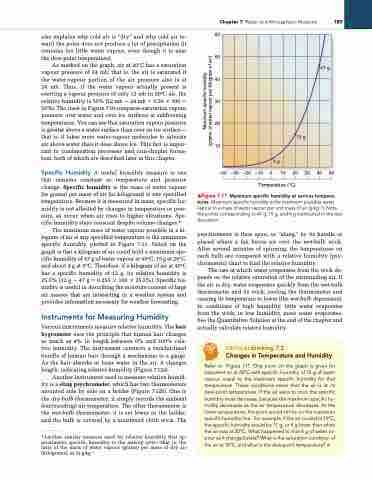
The maximum mass of water vapour possible in a ki logram of air at any specified temperature is the maximum specific humidity, plotted in Figure 7.11. Noted on the graph is that a kilogram of air could hold a maximum spe cific humidity of 47 g of water vapour at 40°C, 15 g at 20°C, and about 4 g at 0°C. Therefore, if a kilogram of air at 40°C has a specific humidity of 12 g, its relative humidity is 25.5% (12 g 4 47 g 5 0.255 3 100 3 25.5%). Specific hu midity is useful in describing the moisture content of large air masses that are interacting in a weather system and provides information necessary for weather forecasting.
Instruments for Measuring Humidity
Various instruments measure relative humidity. The hair hygrometer uses the principle that human hair changes as much as 4% in length between 0% and 100% rela tive humidity. The instrument connects a standardized bundle of human hair through a mechanism to a gauge. As the hair absorbs or loses water in the air, it changes length, indicating relative humidity (Figure 7.12a).
Another instrument used to measure relative humid ity is a sling psychrometer, which has two thermometers mounted side by side on a holder (Figure 7.12b). One is the dry-bulb thermometer; it simply records the ambient (surrounding) air temperature. The other thermometer is the wet-bulb thermometer; it is set lower in the holder, and the bulb is covered by a moistened cloth wick. The
*Another similar measure used for relative humidity that ap proximates specific humidity is the mixing ratio—that is, the ratio of the mass of water vapour (grams) per mass of dry air (kilograms), as in g·kg−1.
60
50
40
30
20
10
–40 –30 –20 –10
Temperature (°C)
40 50
47 g
15 g
4g
0 10 20 30
▲Figure 7.11 Maximum specific humidity at various tempera- tures. Maximum specific humidity is the maximum possible water vapour in a mass of water vapour per unit mass of air (g·kg−1). Note the points corresponding to 47 g, 15 g, and 4 g mentioned in the text discussion.
psychrometer is then spun, or “slung,” by its handle or placed where a fan forces air over the wetbulb wick. After several minutes of spinning, the temperatures on each bulb are compared with a relative humidity (psy chrometric) chart to find the relative humidity.
The rate at which water evaporates from the wick de pends on the relative saturation of the surrounding air. If the air is dry, water evaporates quickly from the wetbulb thermometer and its wick, cooling the thermometer and causing its temperature to lower (the wet-bulb depression). In conditions of high humidity, little water evaporates from the wick; in low humidity, more water evaporates. See the Quantitative Solution at the end of the chapter and actually calculate relative humidity.
CriTiCaLthinking 7.2
Changes in Temperature and Humidity
Refer to Figure 7.11. One point on the graph is given for saturated air at 20°C with specific humidity of 15 g of water vapour, equal to the maximum specific humidity for that temperature. These conditions mean that the air is at its dew-point temperature. if the air were to cool, the specific humidity must decrease, because the maximum specific hu- midity decreases as the air temperature decreases. At the lower temperature, the point would still lie on the maximum specific humidity line. For example, if the air cooled to 10°C, the specific humidity would be 11 g, or 4 g lower than when the air was at 20°C. What happened to that 4 g of water va- pour as it changed state? What is the saturation condition of the air at 10°C, and what is the dew-point temperature? •
Maximum specific humidity
(grams of water vapour per kilogram of air)