Page 351 - Geosystems An Introduction to Physical Geography 4th Canadian Edition
P. 351
Chapter 11 Climate Change 315
440
400
360
320
280
240
200
160
6 4 2 0
–2 –4 –6 –8
–10 –12
1900
1800
1600
1400
1200
1000
800
600
400
200
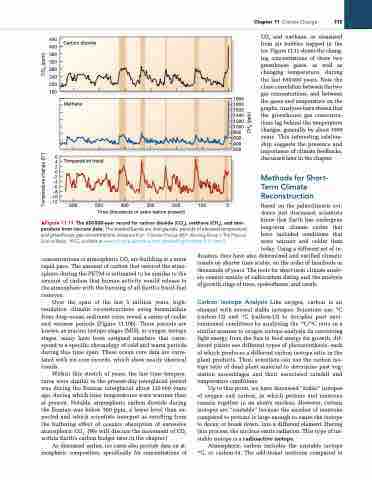
CO2 and methane, as measured from air bubbles trapped in the ice. Figure 11.11 shows the chang- ing concentrations of those two greenhouse gases, as well as changing temperature, during the last 650000 years. Note the close correlation between the two gas concentrations, and between the gases and temperature on the graphs. Analyses have shown that the greenhouse gas concentra- tions lag behind the temperature changes, generally by about 1000 years. This interesting relation- ship suggests the presence and importance of climate feedbacks, discussed later in the chapter.
Methods for Short- Term Climate Reconstruction
Based on the paleoclimatic evi- dence just discussed, scientists know that Earth has undergone long-term climate cycles that have included conditions that were warmer and colder than today. Using a different set of in-
Carbon dioxide
Methane
Temperature trend
600 500 400 300 200 100 0 Time (thousands of years before present)
▲Figure 11.11 The 650 000-year record for carbon dioxide (CO2), methane (CH4), and tem- perature from ice-core data. The shaded bands are interglacials, periods of elevated temperature and greenhouse gas concentrations. [adapted from “Climate Change 2007: Working group i: The Physical Science Basis,” iPCC, available at www.ipcc.ch/publications_and_data/ar4/wg1/en/tssts-2-1-1.html.]
concentrations of atmospheric CO2 are building at a more rapid pace. The amount of carbon that entered the atmo- sphere during the PETM is estimated to be similar to the amount of carbon that human activity would release to the atmosphere with the burning of all Earth’s fossil-fuel reserves.
Over the span of the last 5 million years, high- resolution climatic reconstructions using foraminifera from deep-ocean sediment cores reveal a series of cooler and warmer periods (Figure 11.10b). These periods are known as marine isotope stages (MIS), or oxygen isotope stages; many have been assigned numbers that corre- spond to a specific chronology of cold and warm periods during this time span. These ocean core data are corre- lated with ice-core records, which show nearly identical trends.
Within this stretch of years, the last time tempera- tures were similar to the present-day interglacial period was during the Eemian interglacial about 125000 years ago, during which time temperatures were warmer than at present. Notably, atmospheric carbon dioxide during the Eemian was below 300 ppm, a lower level than ex- pected and which scientists interpret as resulting from the buffering effect of oceanic absorption of excessive atmospheric CO2. (We will discuss the movement of CO2 within Earth’s carbon budget later in the chapter.)
As discussed earlier, ice cores also provide data on at- mospheric composition, specifically for concentrations of
dicators, they have also determined and verified climatic trends on shorter time scales, on the order of hundreds or thousands of years. The tools for short-term climate analy- sis consist mainly of radiocarbon dating and the analysis of growth rings of trees, speleothems, and corals.
Carbon Isotope Analysis Like oxygen, carbon is an element with several stable isotopes. Scientists use 12C (carbon-12) and 13C (carbon-13) to decipher past envi- ronmental conditions by analyzing the 13C/12C ratio in a similar manner to oxygen isotope analysis. In converting light energy from the Sun to food energy for growth, dif- ferent plants use different types of photosynthesis, each of which produces a different carbon isotope ratio in the plant products. Thus, scientists can use the carbon iso- tope ratio of dead plant material to determine past veg- etation assemblages and their associated rainfall and temperature conditions.
Up to this point, we have discussed “stable” isotopes of oxygen and carbon, in which protons and neutrons remain together in an atom’s nucleus. However, certain isotopes are “unstable” because the number of neutrons compared to protons is large enough to cause the isotope to decay, or break down, into a different element. During this process, the nucleus emits radiation. This type of un- stable isotope is a radioactive isotope.
Atmospheric carbon includes the unstable isotope 14C, or carbon-14. The additional neutrons compared to
Temperature change (C°)
CO2 (ppm)
CH4 (ppb)