Page 615 - Geosystems An Introduction to Physical Geography 4th Canadian Edition
P. 615
Chapter 18 The Geography of Soils 579
Ca Mg Ca K +– + +– + –+ – + +
deposition), as discussed in Chapter 3. Scientists have measured acid precipitation values below pH 2.0 (the acidity of lemon juice)—incredibly low for natural pre- cipitation. Increased acidity in the soil solution acceler- ates the chemical weathering of mineral nutrients and increases their depletion rates. Because most crops are sensitive to specific pH levels, acid soils below pH 6.0 require treatment to raise the pH. This soil treatment is accomplished by the addition of bases in the form of minerals that are rich in base cations, usually lime (cal- cium carbonate, CaCO3).
Human Impacts on Soils
Unlike living species, soils do not reproduce, nor can they be re-created. A few centimetres’ thickness of prime farmland soil may require 500 years to mature. Yet this same thickness is being lost annually through soil erosion that occurs when humans remove veg- etation and plough the land, regardless of topography (Figure 18.8). Additional losses occur when flood con- trol structures block sediments and nutrients from replenishing floodplain soils. As a result of human intervention and unsustainable agricultural practices, some 35% of farmlands are losing soil faster than it can form—a loss exceeding 23 billion tonnes per year. Soil depletion (such as the loss of fertility that occurs when soils are leached of cations) and soil loss are at record levels from Iowa to China, Peru to Ethiopia, and the Middle East to the Americas. The impact on soci- ety is potentially disastrous as population and food demands increase.
Soil Erosion
The U.S. NRCS describes soil erosion as “the break- down, detachment, transport, and redistribution of soil particles by forces of wind, water, or gravity.” Over- cultivation and excessive tilling, overgrazing, and the clearing of forested slopes are some of the main human activities that make soils more prone to erosion. Soil erosion removes topsoil, the layer that is richest in organic matter and nutrients. Millennia ago, farmers
Al –––+
– – – – +Na
+++ ++ Mg K CaK
H+
Clay particle
Soil solution
Root hairs
▲Figure 18.6 Soil colloids and cation-exchange capacity (CEC). This typical soil colloid retains mineral ions by adsorption to its sur- face (opposite charges attract). This process holds the ions until they are absorbed by root hairs.
can store or exchange a relatively large amount of cations from the soil solution, an indication of good soil fertility (unless a complicating factor exists, such as a soil that is too acid). Soil is fertile when it contains organic sub- stances and clay minerals that absorb water and adsorb certain elements needed by plants.
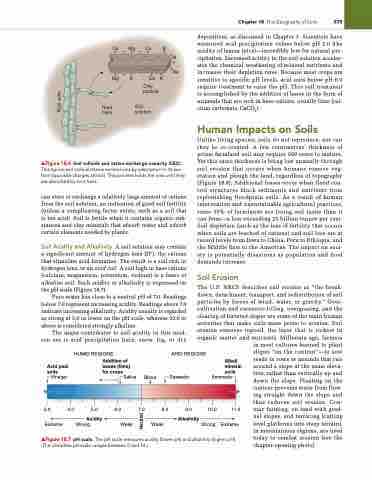
Soil Acidity and Alkalinity A soil solution may contain a significant amount of hydrogen ions (H+), the cations that stimulate acid formation. The result is a soil rich in hydrogen ions, or an acid soil. A soil high in base cations (calcium, magnesium, potassium, sodium) is a basic or alkaline soil. Such acidity or alkalinity is expressed on the pH scale (Figure 18.7).
Pure water has close to a neutral pH of 7.0. Readings below 7.0 represent increasing acidity. Readings above 7.0 indicate increasing alkalinity. Acidity usually is regarded as strong at 5.0 or lower on the pH scale, whereas 10.0 or above is considered strongly alkaline.
The major contributor to soil acidity in this mod- ern era is acid precipitation (rain, snow, fog, or dry
in most cultures learned to plant slopes “on the contour”—to sow seeds in rows or mounds that run around a slope at the same eleva- tion, rather than vertically up and down the slope. Planting on the contour prevents water from flow- ing straight down the slope and thus reduces soil erosion. Con- tour farming, on land with grad- ual slopes, and terracing (cutting level platforms into steep terrain), in mountainous regions, are used today to combat erosion (see the chapter-opening photo).
Acid peat soils
Vinegar
3.0 Extreme
for crops
Saliva
Strong Weak
Blood
7.0 8.0 Weak
Alkali mineral soils
Ammonia
10.0 11.0 Strong Extreme
HUMID REGIONS
Addition of bases (lime)
ARID REGIONS
Seawater
9.0
Alkalinity
4.0
5.0 6.0
Acidity
▲Figure 18.7 pH scale. The pH scale measures acidity (lower pH) and alkalinity (higher pH). (The complete pH scale ranges between 0 and 14.)
Neutral
Root cells